Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 16 October 2020

Risk factors for type 1 diabetes, including environmental, behavioural and gut microbial factors: a case–control study
- Deborah Traversi 1 , 8 ,
- Ivana Rabbone 2 , 7 ,
- Giacomo Scaioli 1 , 8 ,
- Camilla Vallini 2 ,
- Giulia Carletto 1 , 8 ,
- Irene Racca 1 ,
- Ugo Ala 5 ,
- Marilena Durazzo 4 ,
- Alessandro Collo 4 , 6 ,
- Arianna Ferro 4 ,
- Deborah Carrera 3 ,
- Silvia Savastio 3 ,
- Francesco Cadario 3 ,
- Roberta Siliquini 1 , 8 &
- Franco Cerutti 1 , 2
Scientific Reports volume 10 , Article number: 17566 ( 2020 ) Cite this article
7144 Accesses
28 Altmetric
Metrics details
- Microbiology
- Molecular biology
- Risk factors
Type 1 diabetes (T1D) is a common autoimmune disease that is characterized by insufficient insulin production. The onset of T1D is the result of gene-environment interactions. Sociodemographic and behavioural factors may contribute to T1D, and the gut microbiota is proposed to be a driving factor of T1D. An integrated preventive strategy for T1D is not available at present. This case–control study attempted to estimate the exposure linked to T1D to identify significant risk factors for healthy children. Forty children with T1D and 56 healthy controls were included in this study. Anthropometric, socio-economic, nutritional, behavioural, and clinical data were collected. Faecal bacteria were investigated by molecular methods. The findings showed, in multivariable model, that the risk factors for T1D include higher Firmicutes levels (OR 7.30; IC 2.26–23.54) and higher carbohydrate intake (OR 1.03; IC 1.01–1.05), whereas having a greater amount of Bifidobacterium in the gut (OR 0.13; IC 0.05 – 0.34) was a protective factor for T1D. These findings may facilitate the development of preventive strategies for T1D, such as performing genetic screening, characterizing the gut microbiota, and managing nutritional and social factors.
Similar content being viewed by others

Genetic risk for autoimmunity is associated with distinct changes in the human gut microbiome

Functional and metabolic alterations of gut microbiota in children with new-onset type 1 diabetes

Diet, lifestyle and gut microbiota composition among Malaysian women with gestational diabetes mellitus: a prospective cohort study
Introduction.
Type 1 diabetes (T1D) is a multifactor disease caused by β-cell destruction (which is mostly immune-mediated) and absolute insulin deficiency. At present, the management of T1D has been improved, but the disease remains incurable. T1D onset is most common in childhood. T1D represents approximately 5–10% of all diabetes diagnoses 1 . Between 70 and 90% of T1D patients at diagnosis exhibit evidence of an immune-mediated process with β-cell autoantibodies. T1D onset is preceded by a preclinical period that lasts approximately 3 years, in which autoantibodies appear in the circulatory system 2 . Immune destruction of the β-cells can be detected by the evaluation of some haematic markers 3 . The disease has strong HLA associations, which explain nearly half of the genetic disease predisposition, while the remainder is due to other genetic polymorphisms 3 , 4 .
Analysis of genetic disease susceptibility suggests that there is a greater risk of T1D development when the father is affected by the disease than when the mother is affected 5 . On the other hand, there is evidence that a critical role is played by non-genetic factors, including both environmental and host-related factors, which are considered to play decisive roles in the disease process, leading to the manifestation of clinical T1D 6 .
The worldwide incidence of T1D in the age group of 0–15 years varies considerably by region (from 0.5 to 60 per 100,000 children), and the yearly increase ranges from 0.6% to 9.3%. In Europe, the percentage of cases in the age group of 0–15 years will rise by 70% 7 . In the Piedmont region, up to 2013, there were approximately 8,000 cases in this age group with an incidence of 27 new diagnoses per 100,000 8 . Migrant populations tend to show an incidence of diabetes similar to that of most host populations; therefore, a higher T1D incidence in migrant children was observed in Europe 6 , 9 , 10 . Such a pronounced increase in incidence cannot be attributable to genetic factors alone. Other major risk factors may include the environment, Western lifestyle and nutrition 10 . Other diseases with immune involvement, such as allergies, exhibit a similar trend, suggesting an inductor role for exogenous factors regarding the increased predisposition to autoimmunity 11 . Preventive measures to reduce the incidence of T1D have not been defined to date. Various factors seem to be involved in modulating the incidence of T1D, including birth delivery mode, feeding, birth weight, infections (especially viral), dietary behaviour, and pharmaceutical use (especially antibiotics). Such factors may contribute to T1D development during the early disease stage 12 ; however, compared with genetic factors, environmental factors are less well characterized 13 . β- Cell vulnerability to stress factors has been discussed as the basis of the overload hypothesis 14 . Associations among the microbiome, metabolome, and T1D were shown, highlighting a host-microbiota role in the onset of the disease 12 , 15 . The origin of the disease process was suspected to be gut microbiota dysbiosis (imbalances in the composition and function of intestinal microbes) associated with altered gut permeability and a major vulnerability of the immune system 6 . Accordingly, evidence obtained from both animal models and human studies suggests that the gut microbiota and the immune system interact closely, emphasizing the role of the intestinal microbiota in the maturation and development of immune functions 16 . Recently, mycobiome-bacteriome interactions, as well as intestinal virome and islet autoimmunity, were hypothesized to be drivers of dysbiosis 17 . Several studies have specifically investigated microbiota composition in children with T1D 18 , 19 , 20 , but the results have not been consistent. Interestingly, most studies are in agreement regarding the reduced microbial diversity observed in subjects with T1D compared with controls; moreover, the microbiota structure in T1D subjects was found to be different from that of control subjects 21 , 22 . To date, a typical T1D-associated microbiota has not been identified 23 , 24 , 25 , 26 . The research also determined that T1D clinical management could be improved by in-depth analysis of the partial remission phase 27 ; however, preventive measures are limited and generally focus only on genetic susceptibility 28 and general population screening for islet autoimmunity 29 . The development of an integrated prediction strategy could be useful for increasing early diagnosis while avoiding onset complications by identifying children at risk of T1D to place under observation and, in the future, to treat with preventive methods 10 .
The aim of this study is to identify environmental, behavioural, and microbial risk factors of T1D onset to develop an integrated T1D preventive management strategy that is suitable for paediatricians in the Piedmont region.
Subject description and origin factor analysis
To analyse the origin factor, the study population was subdivided by the children's origins (Italian and migrant, 69 and 27 children, respectively). An analysis of the socio-demographic and behavioural factors examined in the study showed many differences between Italian and migrant children, while other variables appear to be quite homogeneous (Table 1 ). In the studied cohort, migrant status did not produce a significant increase in T1D onset.
Approximately 79% of the children in the cohort had siblings; approximately 40% of the included children lived with a pet in the house, and more than 65% of the children took antibiotics during the first two years of life. The residency zone was notably different between Italians and migrants: the percentage of migrant children living in urban sites was higher but not significant following the adjusted model. Regular sports activities seem to be practised more by Italian children than by migrant children (73.5% vs 51.8%, p = 0.054). A total of 77.9% of Italian children and 55.6% of migrant children were subjected to regular health check-ups (p = 0.017). A significant difference was confirmed for the ages of the migrant mother and father (Table 1 ), meanly 6 years and 4 years younger respectively at recruitment, respect the Italians (p = 0.017 and p = 0.0425). The analysis of eating habits and nutritional intake revealed that the majority of the children were breastfed. Moreover, the weaning age was 6 months, as recommended. Migrant children showed higher total carbohydrate intake (+ 12%, p = 0.044) and simple carbohydrate intake (+ 24%, p = 0.0045). Moreover, among migrants, the children tended to access food by themselves and to consume meals alone. The percentage of migrant children who ate meals while watching TV was higher but not significant. Finally, the one-course meal was more frequent in migrant families (ratio 1:3, p = 0.006).
The analysis of microbiota and bioindicator species displayed no significant differences between Italian and migrant children: the qRT-PCR measurements showed a trend of greater value for the total bacteria (both for the experimental design with and without probe), Bacteroides and M. smithii (both using 16S rDNA and nifH) in migrant children. The DGGE profile and dendrogram analysis did not show a different clustering pattern based on the origin, and the migrant group showed a trend towards greater α-diversity of the faecal microbiota profiles (Shannon index + 5%). Additionally, the α-diversity analyses in next generation sequencing (NGS) showed a difference in taxonomic units (OTUs), i.e., there were more OTUs in migrants than in Italians, but the difference was not significant, though it was close to the limit of significance (p = 0.057). Furthermore, the phylogenetic diversity index (Faith PD) suggested that the origin of the subjects could influence the structure of the microbial community. Although the overall number of OTUs did not change significantly, the phylogenetic distance of the individual OTUs was greater in the migrant group than in the Italian group, as the OTUs occupied a broader ecological niche in the migrant group.
T1D risk factors
Previous results indicated that being a migrant child in the Piedmont region is not a significant risk factor for T1D onset 30 . Table 2 shows single logistic regressions performed to estimate the impact of the different variables on the outcome. Notably, the analysis of socio-demographic, behavioural, and nutritional determinants revealed that having parents with at least a high school certificate seems to be a protective factor for T1D onset, even if not significant after adjusted comparisons.
High total caloric intake, as well as high protein intake and consumption of total carbohydrates, are associated with only a slightly increased risk of T1D onset.
The DGGE gel and the results of the cluster analysis are shown in Fig. 1 . The Pearson similarity clustering showed macro beta-diversity differences between the T1D patients and healthy children, with the main division being in two different clusters.
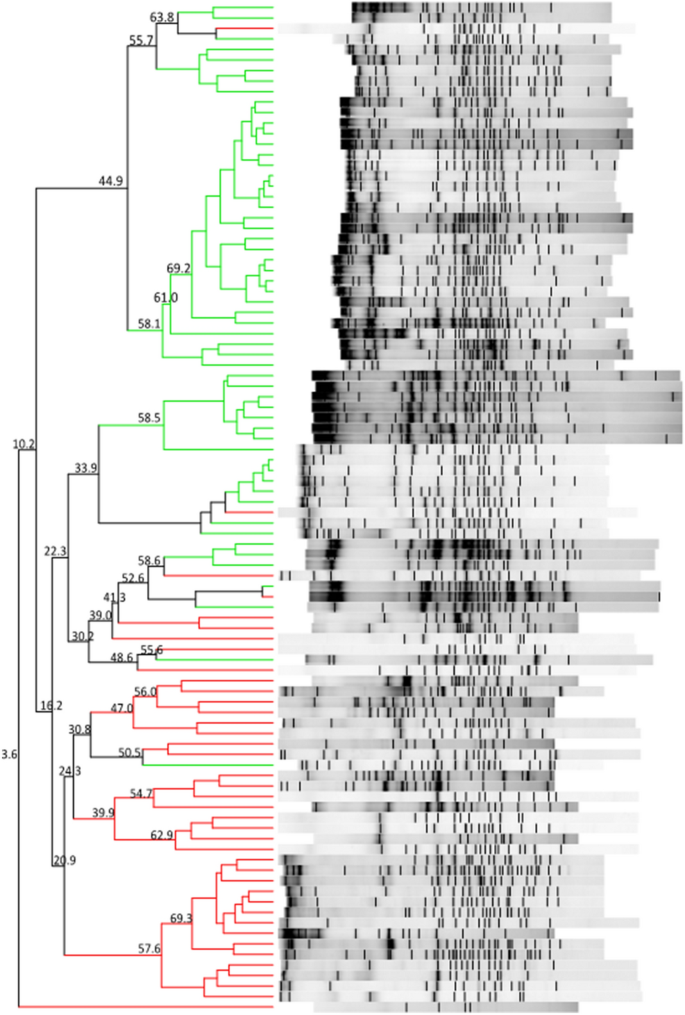
DGGE banding patterns and the results of the analysis in which the Pearson coefficient (numbers reported near the nodes) was used for measuring similarity in banding patterns. The cluster identifies T1D patients (red lines) and healthy children (green lines).
Firmicutes and Bacteroidetes followed by Proteobacteria and Actinobacteria (Table 3 ) predominantly composed the gut microbiota of all children. In the children with diabetes, an increase in the levels of three members of Bacteroidetes ( Alistipes senegalensis , Bacteroides timonensis , and Barnesiella intestinihominis ) and three members of Firmicutes ( Christensenella timonensis ,
Ruminococcus bromii , and Urmitella timonensis ) was observed by sequencing.
Furthermore, other notable results were obtained by NGS analyses. The taxonomic analysis revealed that the gut microbiota of the study participants was composed of nine relevant phyla: Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Verrucomicrobia, Euryarchaeota, Tenericutes, Cyanobacteria, and an unclassified phylum.
Moreover, beta-diversity analyses were carried out to highlight the differences among the samples based on the structures of their microbial communities. The weighted UniFrac metric showed that the samples were not subdivided into clusters. The intragroup and intergroup distances were comparable, and there was no separation between the clusters. These findings were confirmed by the Permanova test. Finally, analyses of the differential abundance were performed to compare the increase or decrease in the abundance of one or more bacteria in the case and control groups. DeSeq2 showed 48 significantly abundant OTUs (p < 0.001). The most abundant OTU was Rikenellaceae followed by Prevotellaceae ( Prevotella copri ), Barnesiellaceae , Lachnospiraceae, and Ruminococcaceae ( Ruminococcus bromii ), which were significantly more abundant in children with diabetes.
The difference in the results observed between methods is an interesting discussion point. The methods are characterized by different sensitivities; they represent different molecular perspectives regarding the faecal microbiota. When a method with a higher sensibility is used (NGS), a flattening effect is possible. On the other hand, the major abundance of such genera as Ruminococcu s was confirmed by different microbiota study methods, which is in keeping with the qRT-PCR results. A group of 23 samples showed different clusterization compared to the others (Fig. 2 , left). This small group was not different from the main group regarding any characteristics. The only significant difference was observed for the M. smithii presence and the A. muciniphila levels, both of which were higher in the separated group (Fig. 2 , right). A. muciniphila was proposed as a probiotic 31 , while M. smithii has been characterized as the most abundant methanogen in the gut 32 .
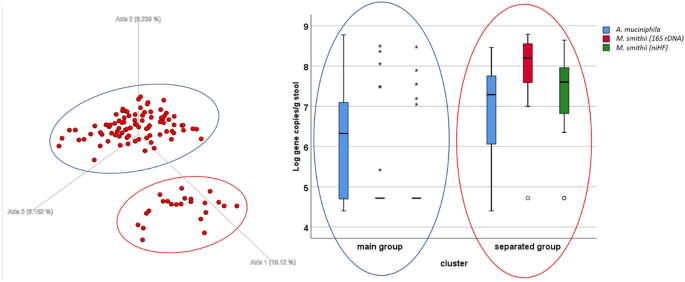
Left-Unweighted UniFrac graph of the NGS results. There are two identifiable groups: the blue circle (main group) and the red circle (separated group). No experimental hypothesis was confirmed for the cluster definition. On the Right: box plot of the qRT-PCR results for some microbiological targets ( Akkermansia muciniphila and Methanobrevibacter smithii ), the difference between the groups is significant (t-test p < 0.05).
The qRT-PCR gut microbiota analysis indicated significant differences among T1D patients and healthy children (Table 2 ). The logistic regression analysis showed that the increase in the Margalef index was associated with a decrease in the likelihood of disease onset (OR 0.20; 95% CI 0.09–0.46, p = 0.000). Increased Firmicutes levels and decreased Bacteroidetes levels were significant risk factors for T1D (OR 7.49; 95% CI 3.25–17.28, p = 0.0001; OR 0.28; 95% CI 0.15–0.51 p = 0.0001, respectively). Moreover, Bifidobacterium spp. was a protective factor for T1D onset (OR 0.20; 95% CI 0.10–0.38, p = 0.0001).
The multivariable analysis produced a R 2 = 0.6259 (p < 0.001). After adjusting for confounding factors, the likelihood of having diabetes is significantly higher in those with higher amount of Firmicutes, lower amount of Bifidobacterium spp and a higher amount of total carbohydrate intake (Table 4 ).
T1D is an important disease that affects health with onset primarily occurring in childhood. At present, there is no cure for this disease, and only disease management is possible. The disease burden of T1D is immense, especially considering the number of years of life lost due to disability but also the years of life lost due to premature death. The life expectancy for T1D patients is approximately 16 years shorter than that of the comparable healthy population 33 . Even if relevant risk factors are known, to date, such scientific determinants do not include a screening programme for preventive purposes. Of course, preventive action must be considered as a systematic process that focuses on the main risk factors to identify children at higher risk of T1D and to suggest efficacious preventive treatments. In the study, the main T1D onset risk factors seem to be identifiable in the composition of the microbiota and, in particular, the microbiota α-diversity, Firmicutes and Bacteroidetes levels and their ratio, as well as the Bifidobacterium level. Similar evidence was obtained by other studies, which observed both higher Bacteroidetes in T1D patients 34 , 35 and less abundant anti-inflammatory genera in children with multiple islet autoantibodies 36 . Reduced microbial diversity appears to become significant between seroconversion and overt T1D 15 . A significant difference in the Bifidobacterium level was observed in different studies, including both a small cohort of autoimmune children 37 , 38 and a larger population associated with such protective factors as breastfeeding 21 . At the genus level, a significant difference in, for example, Blautia (increased in patients), was observed 39 ; however, in other studies, different single species ( Bacteroides ovatus ) seem to be more abundant in patients than in the controls 18 . However, prior studies suggest the presence of duodenal mucosa abnormalities in the inflammatory profile for T1D patients 22 , 40 and on the T1D-related changes in the gut microbiota, even if proving the causality of these factors has remained challenging 21 .
The characterization of the microbiota is rapidly evolving. Traditional methods that are not as sensitive as PCR-DGGE are still suitable, while NGS methods are expanding. Sophisticated whole-genome sequencing methods integrated with metabolomics and proteomics have been proposed. However, the large amount of data, being affected by multiple confounding factors, has not had a clear impact on T1D prevention strategies. The development of a simple method to describe microbiota modulation using validated biomarkers, which could serve as a rapid screening test, may be warranted.
Another risk factor is the occurrence of stress due to a traumatic or emotional experience. This stress seems to be able to affect the autoimmunity process. Therefore, particular attention could be paid to such risk factors for T1D risk in children.
A high education level of one or both parents could be also protective, suggesting that socioeconomic factors affect the T1D risk. Other factors, identified as significant risk modulators among behavioural and nutritional factors, had minor effects.
The study has some potential limitations, including susceptibility to bias in recollection about exposure and reverse causality. The exposure recollection could be biased, but this issue can be less influential at the onset, as in this study. Moreover, recruitment at the onset guarantees a temporal coherence of the exposure with respect to the disease onset.
T1D is one of the most frequently diagnosed diseases in children; however, it is not a high-incidence disease. The prospective inclusion of a large number of healthy children, which is needed for the observation of enough cases, requires a very long time of observation. Moreover, a restricted age range was necessary in children for the rapid changes in behaviour and microbiota. This requirement resulted in an additional included subject restriction. On the other hand, the study of multifactorial diseases with poorly understood pathogenic pathways is imperative, even if it is at risk for obtaining less conclusive evidence. Of course, such a study alone could not elucidate the causation process, but the evidence obtained could be important for the selection of higher-risk subpopulations, planning of future research, and improving prevention.
Identification of a higher-risk subpopulation is strictly relevant for the subsequent validation of an efficient preventive screening to be produced with a prospective method. Of course, the pathogenesis of type 1 diabetes has not been fully elucidated to date; however, in this study, various factors (associated with both the disease and the microbiota composition) were included, such as the origin of the children, the age of the mother, the age of breastfeeding and the age of weaning. Other possible confounding factors not included in our analysis are viral infections, particularly enteroviruses, and preterm birth; however, there was no clear consensus regarding these novel factors at the beginning of the study.
Concerning the microbiota, the knowledge is still incomplete, and various factors can interact to produce a T1D risk modulation that is not explainable at present. Moreover, the results obtained using different techniques were also dissimilar (for example, clusterization due to β-diversity analysis). This finding is likely due to the different sensitivities of the applied methods 41 . Furthermore, even if the time between the symptom comparison and the diagnosis is very short, there is a danger of biased estimates due to reverse causality.
In conclusion, this study confirmed that T1D onset risk is modulated by compositional changes in the gut microbiota and that such evidence must be employed to devise preventive measure. The results showed that the gut microbial indicators found in children with T1D differ from those found in healthy children. These findings also pave the way for new research attempting to develop strategies to control T1D development by modifying the gut microbiota. However, a better knowledge of gut microbial composition associated with the development of T1D must be obtained to choose the best treatment 10 , 42 , 43 , 44 , 45 .
In brief, direct or indirect manipulations of the intestinal microbiome may provide effective measures for preventing or delaying the disease process leading to the manifestation of clinical T1D. At present, a preventive strategy could be developed that includes the main genetic and microbiome risk factors. Then, this strategy could be applied to healthy children to reduce the burden of T1D.
Study design and participants
The case–control study began in January 2016 46 and ended in September 2018 (case–control phase of clinicaltrial.gov Protocol ID: G12114000080001). The work was conducted following the STROBE Statement for a case–control study. The activity is bicentric and includes the two main paediatric hospitals in the Piedmont region (located in Torino and Novara), which cover the clinical management for cases of T1D in the region. The ethics committees of the two hospitals approved the research activities during 2015 (“Comitato etico interaziendale A.O.U. Ordine Mauriziano di Torino ASLTO1” with record number 0117120 and “Comitato etico Interaziendale A.O.U. “Maggiore della Carità” ASL BI, NO, VCO” record number 631/CE).
The recruitment included 40 paediatric patients with T1D (cases) and 56 healthy children (controls), who were comparable in terms of age, gender, and ethnicity to avoid bias. The included subjects represent the most convenient sample possible. The inclusion criteria were age (5–10 years), normal weight, and residence in Piedmont. Exclusion criteria were celiac disease, chronic disease diagnosis, eating disorders, active infections, use of antibiotics and/or probiotics and/or any other medical treatment that influences intestinal microbiota during the 3 months before recruitment and children with parents of mixed origins (Italian and migrant) for the exclusion of important confounding factors due to genetic and cultural mixed backgrounds 19 .
The T1D children were integrated into the study at disease onset, with hyperglycaemia, with or without ketoacidosis, polyuria symptoms, a high value of glycated haemoglobin (HbA1c > 42 mmol/mol) and T1D-specific autoantibody positivity. Healthy children were contacted by paediatricians in the territory of the acute care system. The guardians of the enlisting children read, understood, and then signed informed consent forms following the declaration of Helsinki. A module is prepared for parents, children, and mature children 47 . All the following methods were carried out following relevant guidelines and regulations when available. A questionnaire was given to the parents containing items and questions to retrieve data on the family contest with particular regards to emotive stressors, such as mourning or separation, anthropometrics, and socio-demographic, nutritional, and behavioural information.
Anthropometric and nutritional data included weight, height, body mass index (BMI), food frequency based on 24-h recall and a food frequency questionnaire (FFQ), neonatal feeding, and age of weaning. The anthropometric parameters (weight and height) were measured according to standard recommendations. The BMI values were interpreted according to the WHO criterion. The 24-h recall technique reconstructed the meals and food intake on a recent "typical" day, estimating the bromatological inputs according to a food composition database for epidemiological studies in Italy (BDA). The FFQ, developed for the study, focused on the consumption of certain food categories (those containing sugars, fibre, omega-3, calcium, vitamin D, condiments, and cereals) and eating habits (e.g., alone or with adults, in front of the TV).
Twenty-eight percent of the involved population is migrants (both parents not Italian). Such data are consistent with the percentage of newborns from non-Italian mothers, which is approximately 30% in northern Italy 48 . The migrant group included children coming mainly from northern Africa and Eastern Europe. The migration involved the parents and sometimes the children; on average, the included children as migrants were residents in Italy for less than 5 years. At the end of recruitment, no significant differences were observed between the case and control groups for age, sex composition, and origins (criteria for pairing) or for height, weight, and BMI (T-test, p > 0.05) (Table 5 ).
Sample collection and DNA extraction
A kit for stool collection was delivered to each study participant following a validated procedure 49 , 50 and using a Fecotainer device (Tag Hemi VOF, Netherlands). Faecal samples were homogenized within 24 h in the laboratory, and five 2 g aliquots were stored at − 80 °C until DNA isolation was performed. Total DNA extractions from the stool samples were performed using the QiaAmp PowerFecal DNA Kit (QIAGEN, Hilden, Germany). The nucleic acids were quantified using a NanoQuant Plate (TECAN Trading AG, Switzerland), which allows quantification using a spectrophotometer read at 260 nm. The spectrophotometer used was the TECAN Infinite 200 PRO, and the software was i-Control (version 1.11.10). The extracted DNA concentrations ranged from 1.1–155.5 ng/μl (mean 41.35 ± 38.70 ng/μL). Samples were stored at –20 °C until molecular analysis was performed.
The PCR products for denaturing gradient gel electrophoresis (DGGE) were obtained by amplifying the bacterial 16S rRNA genes following a marker gene analysis approach 51 . The primer pairs were 357F-GC and 518R (Table 6 ) 52 . All PCRs were performed with the T100 Bio-Rad Thermocycler in a 25-μl reaction volume containing 1X Master Mix (166–5009, Bio-Rad, Berkeley, CA, USA), 0.02 bovine serum albumin (BSA), 0.4 μM of each primer, and 2 μl of DNA diluted 1:10 in sterile DNase-treated water. DGGE was carried out using a DCode System (Bio-Rad) with a 30–50% denaturing gradient of formamide and urea 53 . Electrophoresis ran at 200 V for 5 h at 60 °C in 1X TAE buffer. Gels were stained for 30 min with SYBR Green I nucleic acid gel stain (10.000X in DMSO, S9430, Sigma-Aldrich, USA) and were visualized using the D-Code XR apparatus from Bio-Rad. Then, DGGE bands were excised, incubated overnight at − 20 °C, washed, and crushed in 20 μl of molecular-grade water. The supernatant (2 μl) was used as a template and reamplified, as previously described, without BSA and using modified linker-PCR bacterial primers (357F-GC; 518R-AT-M13) (Table 6 ) 19 , 52 , 54 , 55 , 56 , 57 , 58 , 59 , 60 . The obtained PCR products were sequenced with Sanger sequencing (Genechron-Ylichron S.r.l.). The sequence similarities were obtained by the National Centre for Biotechnology Information (NCBI) database using nucleotide Basic Local Alignment Search Tool (BLASTn) analysis.
High-throughput DNA sequencing and analysis were conducted by BMR Genomics s s.r.l. The V3-V4 region of 16S rDNA was amplified using the MiSeq 300PEPro341F and Pro805R primer pair 6 . The sample reads were above 12*10 6 . The reaction mixture (25 μl) contained 3–10 ng/μl genomic DNA, Taq Platinum HiFi (Invitrogen, Carlsbad, CA), and 10 μM of each primer. The PCR conditions for amplification of DNA were as follows: 94 °C for 1 min (1X), 94 °C for 30 s, 55 °C for 30 s, 68 °C for 45 s (25X), and 68 °C for 7 min (1X). PCR products were purified through Agencourt XP 0.8X Magnetic Beads and amplified shortly with the Index Nextera XT. The amplicons were normalized with SequalPrep (Thermo Fisher) and multiplexed. The pool was purified with Agencourt XP 1X Magnetic Beads, loaded onto MiSeq, and sequenced with the V3 chemistry-300PE strategy.
Starting from the extracted DNA, the following microbial targets were quantified by qRT-PCR using a CFX Touch Real-Time PCR Detection System (Bio-Rad-Hercules, CA) and CFX Manager (3.1 Software): total Bacteria, Bacteroidetes, Bacteroides spp., Firmicutes, Bifidobacterium spp., Akkermansia muciniphila, and Methanobrevibacter smithii . Total bacteria and M. smithii were detected following two reaction designs. For M. smithii , the analysis was performed using as target both the 16S rDNA and then a specific functional gene ( nifH ). For total bacteria, quantification was carried out using a protocol with or without a probe. For the determination of total bacteria (method without probe), Bacteroidetes, Bacteroides spp., Firmicutes, Bifidobacterium spp. and Akkermansia muciniphila , 2 µl of 1:10 extracted DNA was added to a reaction mixture consisting of 10 µl Sso Advance SYBR Green Supermix (172–5261, Bio-Rad), 0.5 µl each of the forward and reverse primers (10 µM final concentration) and 7 µl of ultrapure water in a 20 µl final reaction volume. The reaction conditions were set as follows: 95 °C for 3 min (1X), 95 °C for 10 s, and 59 °C for 15 s (57 °C for Bacteroidetes spp. and 60 °C for Firmicutes), 72 °C for 10 s (39X), 65 °C for 31 s, 65 °C for 5 s + 0.5 °C/cycle, ramp 0.5 °C/s (60X). Moreover, for the determinations of M. smithii and total bacteria (method with probe), the reaction was as follows. Two microlitres of 1:10 extracted DNA was added to a reaction mixture consisting of 10 µl IQ Multiplex PowerMix (Bio-Rad-Hercules, CA), 0.2 µl of the molecular probe (10 µM), 0.5 µl each of the forward and reverse primers (10 µM final concentration) and 6.8 µl of ultrapure water in a 20 µl final reaction volume. The reaction conditions were 95 °C for 3 min (1X), 95 °C for 10 s, 59 °C for 15 s, 72 °C for 15 s (39X), and 72 °C for 5 min. Standard curves were produced with serial six-fold dilutions of genomic DNA from the microorganism target, provided by ATCC (Manassas, Virginia, USA) or DSMZ (Braunschweig, Germany). All PCR tests were carried out in triplicate. Table 6 provides detailed information regarding oligonucleotide sequences and genomic standards 19 , 54 , 55 , 56 , 57 , 58 , 59 , 60 . The PCR efficiencies were always between 90 and 110%. To confirm the amplification of each target, gel electrophoresis was performed on 2% agarose gels.
Data elaboration and statistical analyses
The statistical analysis was performed using STATA version 11.0. Moreover, the data on the included T1D patients and healthy controls were elaborated to highlight the likelihood of having diabetes. A descriptive analysis of the variables was conducted. The data were reported as absolute numbers and percentages for categorical variables and as means and standard deviations for continuous variables. Moreover, the subjects were divided by individual origins into two groups: Italian and migrant, considering the origin of the children and their families, to show differences in the distribution of disease determinants and to assess whether being a migrant could be associated with T1D onset. Differences between Italian and migrant children were assessed using the χ 2 test with Fisher’s correction for categorical variables and Student’s t-test for continuous variables. Univariable logistic regression was then performed to estimate the impact of sociodemographic, nutritional, and microbiota-related variables on the outcome. These associations were expressed as odds ratios (OR) at a 95% confidence interval (CI). Moreover, the adjusted p-value for multiple comparisons was calculated using the Benjamini and Hochberg false discovery rate method. We conducted multivariable analyses including various variables (age, gender, Firmicutes, Bifidobacterium spp ., and total carbohydrate intake) and the risk of type 1 diabetes using logistic regression models. The Spearman rank-order correlation coefficient was also determined to assess the relationships between variables. A p-value p < 0.05 was considered significant for all analyses.
The DGGE gel analysis was performed with Bionumerics 7.2. The hierarchical classification was performed with a UPGMA system (1% tolerance and optimization level) and Pearson correlation. Simpson's diversity index, Shannon’s index, and Margalef index were calculated for each DGGE profile to evaluate alpha diversity.
NGS bioinformatics analysis was performed with the software pipeline Qiime2. The reads were cleaned up by the primers using the software Cutadapt (version 2018.8.0) and processed with the software DADA2. The sequences were trimmed at the 3′ end (forward: 270 bp; reverse 260 bp), filtered by quality, and merged with default values. Subsequently, the sequences were elaborated to obtain unique sequences. In this phase, the chimaeras (denoised-paired) are also eliminated. The sequences were clustered against unique sequences at 99% similarity. The taxonomies of both GreenGenes (version 13–8) and Silva (version 132) were assigned to the OTU sequences. Alpha-diversity analyses were performed on all samples using the observed OTUs, Shannon, Pielou's evenness, and Faith PD indices, and for each index, the Kruskal–Wallis test was used to verify the significance of the comparisons between samples. Beta-diversity analyses were performed on all samples using the Bray–Curtis, Jaccard, and UniFrac metrics (weighted and unweighted). Multivariable statistical analyses were performed using the PERMANOVA, Adonis, and ANOSIM tests; instead, the analysis of the differential abundance was based on the packages of R (MetagenomeSeq, DeSeq2, and ANCOM).
Data availability
The database includes human data that are available upon reasonable request.
National Center for Chronic Disease Prevention and Health Promotion. National Diabetes Statistics Report, 2017. Estimates of Diabetes and Its Burden in the United States. CDC (2017).
Mikael Knip, Md, P. et al. Prediction of Type 1 Diabetes in the General Population. Diabetes Care 33 , 1206–1212 (2010).
American Diabetes Association. Standard medical care in diabetes - 2018. Diabetes Care 41 , 1–159 (2018).
Article Google Scholar
Pociot, F. & Lernmark, Å. Genetic risk factors for type 1 diabetes. Lancet 387 , 2331–2339 (2016).
Article CAS PubMed Google Scholar
Turtinen, M. et al. Characteristics of familial type 1 diabetes : effects of the relationship to the affected family member on phenotype and genotype at diagnosis. (2019).
Knip, M., Luopajärvi, K. & Härkönen, T. Early life origin of type 1 diabetes. Semin. Immunopathol. 39 , 653–667 (2017).
World Health Organization. Global Report on Diabetes . (2016).
Bruno, G. Il registro diabete Piemonte. Ital. Heal. Policy Br. 1–8 (2016).
Regnell, S. E. & Lernmark, Å. Early prediction of autoimmune (type 1) diabetes. Diabetologia 60 , 1370–1381 (2017).
Knip, M. & Honkanen, J. Modulation of type 1 diabetes risk by the intestinal microbiome. Curr. Diab. Rep. 17 , 4–11 (2017).
Article CAS Google Scholar
Bach, J.-F. & Chatenoud, L. The hygiene hypothesis : an explanation for the increased frequency of insulin. Cold Sping Harb. Perpect. Med. 2 , a007799 (2012).
Google Scholar
Zununi Vahed, S., Moghaddas Sani, H., Rahbar Saadat, Y., Barzegari, A. & Omidi, Y. Type 1 diabetes: through the lens of human genome and metagenome interplay. Biomed. Pharmacother. 104 , 332–342 (2018).
Butalia, S., Kaplan, G. G., Khokhar, B. & Rabi, D. M. Environmental risk factors and type 1 diabetes: past, present, and future. Can. J. Diabetes 40 , 586–593 (2016).
Article PubMed Google Scholar
Ilonen, J., Lempainen, J. & Veijola, R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat. Rev. Endocrinol. 15 , 635–650 (2019).
Siljander, H., Honkanen, J. & Knip, M. Microbiome and type 1 diabetes. EBioMedicine 46 , 512–521 (2019).
Hooper, L. V., Littman, D. R., Macpherson, A. J. & Program, M. P. Interactions between the microbiota and the immune system. 336 , 1268–1273 (2012).
CAS Google Scholar
Davis-richardson, A. G. & Triplett, E. W. On the role of gut bacteria and infant diet in the development of autoimmunity for type 1 diabetes. Reply to Hänninen ALM and Toivonen RK [ letter ]. 2197–2198 (2015). doi: https://doi.org/10.1007/s00125-015-3701-x
Giongo, A. et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 5 , 82–91 (2011).
Murri, M. et al. Gut microbiota in children with type 1 diabetes differs from that in healthy children : a case-control study. 1–12 (2013).
Mejìa-Leòn, M. E., Petrosino, J. F., Ajami, N. J., Domìnguez-Bello, M. G. & Calderòn de la Barca, M. Fecal microbiota imbalance in Mexican children with type 1 diabetes. 4 , 1–5 (2013).
Stewart, C. J. et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 562 , 583–588 (2018).
Article ADS CAS PubMed Google Scholar
Vatanen, T. et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature 562 , 589–594 (2018).
De Goffau, M. C. et al. Fecal Microbiota Composition Differs Between Children With Beta-Cell Autoimmunity and Those Without. Diabetes 62 , 1238–1244 (2013).
Article PubMed CAS Google Scholar
Davis-Richardson, A. G. et al. Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front. Microbiol. 5 , 1–11 (2014).
Kostic, A. D. et al. The Dynamics of the Human Infant Gut Microbiome in Development and in Progression toward Type 1 Diabetes. Cell Host Microbe 17 , 260–273 (2015).
Article MathSciNet CAS PubMed Google Scholar
Kemppainen, K. M. et al. Early childhood gut microbiomes show strong geographic differences among subjects at high risk for type 1 diabetes. Diabetes Care 38 , 329–332 (2015).
Zhong, T. et al. The remission phase in type 1 diabetes: changing epidemiology, definitions and emerging immuno-metabolic mechanisms. Diabetes Metab. Res. Rev. https://doi.org/10.1002/dmrr.3207 (2019).
Winkler, C. et al. Identification of infants with increased type 1 diabetes genetic risk for enrollment into Primary Prevention Trials—GPPAD-02 study design and first results. Pediatr. Diabetes https://doi.org/10.1111/pedi.12870 (2019).
Ziegler, A.-G. et al. Screening for asymptomatic β-cell autoimmunity in young children No Title. Lancet Child Adolesc. Heal. May , 288–290 (2019).
Rabbone, I. et al. Microbiota, epidemiological and nutritional factors related to ketoacidosis at the onset of type 1 diabetes. Acta Diabetol. https://doi.org/10.1007/s00592-020-01555-z (2020).
Cani, P. D. Human gut microbiome: hopes, threats and promises. Gut 67 , 1716–1725 (2018).
Dridi, B., Raoult, D. & Drancourt, M. Archaea as emerging organisms in complex human microbiomes. Anaerobe 17 , 56–63 (2011).
Rawshani, A. et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet 392 , 477–486 (2018).
Mejía-León, M. E., Petrosino, J. F., Ajami, N. J., Domínguez-Bello, M. G. & De La Barca, A. M. C. Fecal microbiota imbalance in Mexican children with type 1 diabetes. Sci. Rep. 4 , 1–5 (2014).
Alkanani, A. K. et al. Alterations in intestinal microbiota correlate with susceptibility to type 1 diabetes. Diabetes 64 , 3510–3520 (2015).
Harbison, J. E. et al. Gut microbiome dysbiosis and increased intestinal permeability in children with islet autoimmunity and type 1 diabetes: a prospective cohort study. Pediatr. Diabetes 20 , 574–583 (2019).
CAS PubMed Google Scholar
Maffeis, C. et al. Association between intestinal permeability and faecal microbiota composition in Italian children with beta cell autoimmunity at risk for type 1 diabetes. 700–709 (2016). doi: https://doi.org/10.1002/dmrr
Murri, M. et al. Association between intestinal permeability and faecal microbiota composition in Italian children with beta cell autoimmunity at risk for type 1 diabetes. 1–12 (2013). doi: https://doi.org/10.1002/dmrr
Qi, C. J. et al. Imbalance of fecal microbiota at newly diagnosed type 1 diabetes in Chinese Children. 129 , 1298–1304 (2016).
Pellegrini, S. et al. Duodenal mucosa of patients with type 1 diabetes shows distinctive inflammatory profile and microbiota. J. Clin. Endocrinol. Metab. 102 , 1468–1477 (2017).
Putignani, L., Del Chierico, F., Petrucca, A., Vernocchi, P. & Dallapiccola, B. The human gut microbiota: a dynamic interplay with the host from birth to senescence settled during childhood. Pediatr. Res. 76 , 2–10 (2014).
Regueiro, L. et al. Relationship between microbial activity and microbial community structure in six full-scale anaerobic digesters. Microbiol. Res. 167 , 581–589 (2012).
Uusitalo, U. et al. Association of Early Exposure of Probiotics and Islet Autoimmunity in the TEDDY Study. JAMA Pediatr. 33612 , 1–9 (2015).
Panigrahi, P. Probiotics and prebiotics in neonatal necrotizing enterocolitis: New opportunities for translational research. Pathophysiology 21 , 35–46 (2014).
Brüssow, H. Biome engineering-2020. Microb. Biotechnol. 9 , 553–563 (2016).
Traversi, D. et al. Gut microbiota diversity and T1DM onset: Preliminary data of a case-control study. Hum. Microbiome J. 5–6 , 11–13 (2017).
World Health Organization. ICF Parental Consent-clinicalstudies. (2018).
Ministero della Salute. Certificato di assistenza al parto (CeDAP). Analisi dell’evento nascita - Anno 2015 . (2018).
Franzosa, E. A. et al. Relating the metatranscriptome and metagenome of the human gut. PNAS 111 , E2329–E2338 (2014).
IHMS Consortium. IHMS-SOP 02 V2: Standard Operating Procedure for Fecal Samples Self ‐ Collection Laboratory Analysis Handled Within 4 To 24 Hours . (2015).
Knight, R. et al. Best practices for analysing microbiomes. Nat. Rev. Microbiol. 16 , (2018).
Muyzer, G., Waal, E. C. D. E. & Uitierlinden, A. G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59 , 695–700 (1993).
Article CAS PubMed PubMed Central Google Scholar
Webster, N. S. & Negri, A. P. Site-specific variation in Antarctic marine biofilms established on artificial surfaces. Environ. Microbiol. 8 , 1177–1190 (2006).
Dridi, B., Henry, M., El Khechine, A., Raoult, D. & Drancourt, M. High prevalence of Methanobrevibacter smithii and Methanosphaera stadtmanae detected in the human gut using an improved DNA detection protocol. PLoS ONE 4 , e7063 (2009).
Article ADS PubMed CAS Google Scholar
Dao, M. C. et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity : relationship with gut microbiome richness and ecology. Gut Microbiota 65 , 426–436 (2016).
Guo, X. et al. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett. Appl. Microbiol. 47 , 367–373 (2008).
Johnston, C., Ufnar, J. A., Griffith, J. F., Gooch, J. A. & Stewart, J. R. A real-time qPCR assay for the detection of the nifH gene of Methanobrevibacter smithii, a potential indicator of sewage pollution. J. Appl. Microbiol. 109 , 1946–1956 (2010).
Matsuki, T. et al. Quantitative PCR with 16S primers for analysis of human intestinal bifidobacteria. Appl. Environ. Microbiol. 70 , 167–173 (2004).
Nakayama, T. & Oishi, K. Influence of coffee ( Coffea arabica ) and galacto-oligosaccharide consumption on intestinal microbiota and the host responses. FEMS Microbiol. Lett. 343 , 161–168 (2013).
Takahashi, S., Tomita, J., Nishioka, K., Hisada, T. & Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of bacteria and archaea using next-generation sequencing. PLoS ONE 9 , 1–9 (2014).
Download references
Acknowledgements
The authors are grateful to the Italian Ministry of Health (RF-2011-02350617), the University of the Study of Torino and the Città della salute e e della scienza di Torino and the Hospital “Maggiore della Carità" di Novara for co-funding this project. Moreover, the authors wish to thank dr. Barbara Di Stefano (Sanitary Direction AOU Novara) and Mrs Rim Maatoug, Mrs Shpresa Xheka, and Mrs Daniela Elena Zelinschi (cultural intermediaries) at Novara Hospital for the translation of the questionnaire for migrant people. Finally, the authors make a special acknowledgement to the participant children and their families.
Author information
Authors and affiliations.
Department of Public Health and Pediatrics, University of Turin, Piazza Polonia 94, 10126, Torino, Italy
Deborah Traversi, Giacomo Scaioli, Giulia Carletto, Irene Racca, Roberta Siliquini & Franco Cerutti
S.S.V.D. Endocrinology and Diabetology, O.I.R.M., Azienda Ospedaliera Città Della Salute E Della Scienza, Turin, Italy
Ivana Rabbone, Camilla Vallini & Franco Cerutti
Paediatric Endocrinology, Azienda Ospedaliero Universitaria Maggiore Della Carità - Novara, Novara, Italy
Deborah Carrera, Silvia Savastio & Francesco Cadario
S.C.U. Medicina Interna 3, Azienda Ospedaliera Città Della Salute e Della Scienza Di Torino, Torino, Italy
Marilena Durazzo, Alessandro Collo & Arianna Ferro
Department of Veterinary Sciences, University of Turin, Torino, Italy
Dietetic and Clinical Nutrition Department, Azienda Ospedaliero Universitaria Maggiore Della Carità, Novara, Italy
Alessandro Collo
Department of Health Science, University of Eastern Piedmont Amadeo Avogadro - Azienda Ospedaliero Universitaria Maggiore Della Carità - Novara, Novara, Italy
Ivana Rabbone
Department of Public Health and Pediatrics, Hygiene Unit, University of the Study of Turin, via Santena 5 bis, 10126, Torino, Italy
Deborah Traversi, Giacomo Scaioli, Giulia Carletto & Roberta Siliquini
You can also search for this author in PubMed Google Scholar
Contributions
F.C. and R.S. coordinate the work. F.C., I.R., R.S., D.T.: design the work. F.C., I.R., S.S., and F.C.: patient inclusion and questionnaire administration. C.V., D.C.: clinical data collection, Torino and Novara, respectively. I.R.: patient sample collection and transport, questionnaire elaboration. D.T., G.C.: sample processing and extraction, molecular analysis. G.S., U.A., D.T. : statistical analysis and bioinformatics. M.D., A.C., A.F.: nutritional data elaboration. G.C., G.S.: drafted the work. F.C., I.R., R.S., M.D.: revised the work. D.T.: substantively revised the work.
Corresponding author
Correspondence to Deborah Traversi .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Traversi, D., Rabbone, I., Scaioli, G. et al. Risk factors for type 1 diabetes, including environmental, behavioural and gut microbial factors: a case–control study. Sci Rep 10 , 17566 (2020). https://doi.org/10.1038/s41598-020-74678-6
Download citation
Received : 22 January 2020
Accepted : 30 September 2020
Published : 16 October 2020
DOI : https://doi.org/10.1038/s41598-020-74678-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Clin Case Rep
- v.9(11); 2021 Nov
A case report: First presentation of diabetes mellitus type 1 with severe hyperosmolar hyperglycemic state in a 35‐month‐old girl
1 Division of Pediatric Intensive Care, Department of Pediatrics, Shiraz University of Medical Sciences, Shiraz Iran
Homa Ilkhanipoor
2 Division of Pediatric Metabolism and Endocrinology, Department of Pediatrics, Shiraz University of Medical Sciences, Shiraz Iran
Associated Data
All data generated or analyzed during this study are included in this published article.
Hyperglycemic hyperosmolar syndrome (HHS) is a rare complication of diabetes mellitus among pediatric patients. Since its treatment differs from diabetic ketoacidosis (DKA), hence, pediatricians should be aware of its diagnosis and management.

1. BACKGROUND
Hyperglycemic hyperosmolar syndrome (HHS), which is characterized by increased serum glucose concentrations and hyperosmolality with low or absence of ketonemia or ketonuria, has been rarely reported in children. Herein, we report a 35‐month‐old girl, who was newly diagnosed with diabetes mellitus type1 (T1DM), with presentation of HHS that developed some complications.
Hyperosmolar hyperglycemic state (HHS), a rare diabetic hyperglycemic emergency, is most often observed in adult patients, but seldom seen in pediatric patients. Nevertheless, it can present in younger adults and teenagers as the first presentation of diabetes mellitus type 2 (T2DM). 1
HHS is diagnosed by the following criteria: plasma glucose more than 600 mg/dl, venous pH > 7.25, serum bicarbonate >15 mmol/L, small amount of ketonuria or its absence, effective serum osmolality >320 mOsm/kg, and obtundation, combativeness, or seizures (in approximately 50% of all cases). 1 , 2
The incidence of HHS has merely been reported in 0.8–2% of all pediatric patients, 3 , 4 but it has a higher mortality rate in children compared to DKA (~10–35%). 5
Among the precipitating factors for HHS, the main one is infection which has to be diagnosed and treated immediately.
DKA mostly develops within hours of its onset, and the main presentations are as follows: hyperventilation, vomiting, and abdominal pain which force the parents to take their children to a physician. On the contrary, HHS develops over several days and presents itself later on. In this case, patients have polyuria and polydipsia for a longer period; hence, it might not be recognizable, and ultimately present itself with severe dehydration and electrolyte disturbance. It should be noted that the degree of dehydration, electrolyte, and metabolic disturbances are more severe.
Although in HHS there is substantial loss of electrolyte, and volume, the signs of dehydration are not recognizable, due to either obesity or hypertonicity. Hence, clinical assessment of dehydration becomes more difficult. Moreover, treatment of children with HHS differs from DKA; as in patients with HHS, intravascular volume should be replaced more and faster compared to DKA in order to avoid vascular collapse.
The recommendations in the treatment of HHS in pediatric patients are based on adult experiences. The first step is fluid therapy in order to expand intra‐ and extravascular fluid to preserve renal perfusion; hence, the rate of hydration becomes much faster than DKA. 1 , 2
Nonetheless, there are some HHS’ related complications that can be life‐threatening, such as vascular complications (eg, myocardial infarction, stroke, and peripheral arterial thrombosis), central nervous system complications (eg, seizures, cerebral edema, and central pontine myelinolysis (CPM)), which are uncommon, but described as HHS complications. 1 , 2
In this case report, a 3‐year‐old girl is presented with HHS who developed some complications.
2. CASE PRESENTATION
A previously healthy 35‐month‐old girl was brought to the emergency room of the Namazi hospital, Shiraz, Iran, due to reduced level of consciousness. She was well up to five days prior to her admission, after that she presented with dysuria and loss of appetite, and then developed polyuria, polydipsia, and weight loss (14 kg → 11 kg). There was no history of DM in her family.
On arrival, her height was measured 92 cm (25th−50th) percentile), she weighed 11 kg (5th–10th) percentile), and her Body Mass Index (BMI) (BMI‐for‐age) was at the 72nd percentile. Her vital signs were as follows: temperature: 38°C, heart rate (HR): 160, blood pressure (BP): 95/50, and her Glasgow coma scale (GCS) was 11/15. During her physical examination, capillary refilling time was more than 3 s with weak pulses. When checked with a capillary blood glucose meter, her glucose level was too high, so a serum glucose test was performed. Moreover, her first VBG was as follows: pH 7.26, PCO 2 : 32, PO 2 : 39, HCO 3 : 15.8, and BE: −12.6. Due to the decreased level of consciousness, brain CT scan was performed in which brain edema was reported.
As the first line of treatment, she was hydrated with normal saline (10 cc per kg), then the laboratory report revealed; blood sugar: 1124 mg/dl, BUN: 71 mg/dl, creatinine: 1.9 mg/dl, Na: 170 mEq/L (corrected Na: 186), and K: 5.1 mEq/L (effective osmolality was 402). Additionally, urine analysis was SG: 1.010, GLU: 3+, Ketone: trace. VBG after first hydration was as follows: pH: 7.27, PCO 2 : 34.8, and HCO 3 : 15.8. Based on the laboratory report; HHS was confirmed as diagnosis; hence, she was transferred to the Pediatric Intensive Care Unit (PICU).
On arrival at PICU, she developed generalized convulsion, and her GCS declined to less than 8; so she was intubated.
She was hydrated with normal saline again; then, the intravenous fluid was administered with 15 percent deficit and maintenance of fluid in addition to urine output replacement. Due to brain edema, deficit was given over 72 h, but according to urine output and serum sodium level, the amount of deficit increased up to 18 percent, and the sodium content of IV fluid decreased.
The first sodium level reported in PICU was 185 mEq/L, but it gradually decreased as hydration continued, and the amount of fluid increased (with the target level of 10 mEq/L decrease per day); then, it became stable around 145 mEq/L over the next five days. Our primary goal in her treatment was to correct sodium level maximum 10 mEq/L per day, to decrease osmolality maximum 3–5 mosm/L/h, and to reduce blood sugar maximum 75 mg/dl per hour.
On the 3rd day, HHS was resolved, but the patient became febrile, and the amount of endotracheal tube (ETT) secretion increased. The culture of ETT secretions revealed candida non‐albicans and pseudomonas aeruginosa; hence, antibiotic was prescribed. Subsequently, the patient developed hypotension, so inotrope was initiated. On the same day, her blood creatine phosphokinase (CPK) increased to 6400 IU/L, and it reached 13,400 on the fifth day. Then, she developed hemoglobinuria; thereafter, hydration continued until the CPK level returned normal level on the 18th day (Table 1 ).
Laboratory findings
| Tests | Results (day 1) | Day 4 | Day 14 | |
|---|---|---|---|---|
| Sodium (135–145 mEq/L) | 170 | 154 | 138 | |
| Potassium (3.5–5.1 mEq/L) | 5.1 | 3.4 | 4.7 | |
| Chloride | 95 | 128 | 105 | |
| Phosphorus (3.4–4.5 mg/dl) | 3.1 | 1.2 | 4 | |
| Serum glucose (mg/dl) | 1124 | 220 | 180 | |
| Blood urea nitrogen | 71 | 18 | 9 | |
| Creatinine | 1.9 | 0.6 | 0.4 | |
| Venous blood gas | pH | 7.26 | 7.40 | |
| HCO | 15.8 | 24 | ||
| Effective osmolality (275–295 mOsm/kg) | 402 | |||
| Urine analysis (ketone) | +/− | Negative | Negative | |
| CPK | 212 | 6400 | 256 | |
| Magnesium (1.7–2.2 mg/dl) | 3.1 | 1.5 | 2.2 | |
| Calcium (8.5–10.5 mg/dl) | 10 | 8.7 | 10 | |
| CRP (<5) | 4 | >150 | 3 | |
Although enoxaparin was initiated for deep venous thrombosis (DVT) prophylaxis, she presented with the left forearm and hand swelling, on the 6th day. Doppler ultrasonography revealed thrombosis of the distal part of the brachial artery, so the therapeutic dose was initiated. Consequently, her conditions improved after 2 days, and a week later, Doppler sonography was normal.
On the 9th day, she was weaned off the ventilator, and on the 21st day, the patient was discharged from hospital without any sequela.
3. DISCUSSION AND CONCLUSIONS
Hyperglycemic hyperosmolar syndrome (HHS) is a rare presentation of DM in pediatric patients, especially as the first presentation of T1DM, with a high mortality rate. HHS is not easy to diagnose according to physical examination, patient's past medical history or even with availability of laboratory data. However, physicians do not need to be too concerned of HHS in young children, especially if the patient is not obese. 5
Contrary to the frequent symptoms of DKA, such as vomiting, abdominal pain, and drowsiness that force parents to refer to a hospital, the gradual increase of HHS symptoms can cause delayed referral which can ultimately result in severe dehydration and electrolyte imbalance. As a result, proper diagnosis can lead to appropriate management.
HHS generally occurs among obese people and in T2DM, 6 but there are some rare reports on HHS in non‐obese patients and HHS in T1DM. 7 It should be noted that our patient had a normal BMI and T1DM.
Fluid deficits in HHS patients are frequently 12–15% that has to be corrected gradually and uniformly over 24–48 h, but it can be increased up to 20% or more to gradually decline serum sodium and osmolality. 1 , 2
At present time, there is no standard therapeutic guideline for HHS in children. Nonetheless, the two most important points in HHS management are fluid replacement, and gradually reduction of serum osmolality and sodium level. Fluid replacement in children with HHS should be carried out more swiftly with more amount of fluid in comparison with children with DKA. To gradually reduction of hypernatremia, we constantly measured her serum sodium level in order to adjust fluid sodium concentration. Due to the presence of brain edema, reaching the aforementioned goals became more difficult and required more attention.
The insulin infusion strategy might differ from insulin infusion rate, which is 0.1 unit/kg/h in patients with DKA, whereas it should be 0.025–0.05 unit/kg/h in patients with HHS. 1 Our patient was first diagnosed with DKA and treated as DKA in the emergency room; she was hydrated with 10 cc/kg normal saline, and deficit volume was estimated 10%; Insulin treatment was initiated, but after 3 h sodium level increased to 185 mEq/L. According to HHS protocol in PICU, insulin infusion was stopped and the patient hydrated up to 40 cc/kg with normal saline till the patient's hemodynamic became stable. Then, after the initial rehydration, insulin infusion was initiated with 0.03 units per kg per hour, and after 8 h, the sodium content of IV fluid was steadily reduced to 100 mEq/L.
Initially, the degree of dehydration was estimated 15%, but according to serum osmolality, serum sodium, and urine out, the percentage of deficit had increased to 18%.
There are some serious complications in HHS, for example, brain edema, arterial and venous thrombosis, and rhabdomyolysis. 8 In our patient, the complications were brain edema, rhabdomyolysis, and arterial thrombosis (distal part of brachial artery). The patients with HHS are at risk of venous thrombosis, especially those who are immobile more than 48 h, and for those who central venous catheter is inserted. 1 , 8 Although at the beginning of PICU admission we started enoxaparin for prophylaxis, she presented arterial thrombosis.
Altered level of consciousness is commonly seen in adult patients with osmolality more than 330 mOsm/kg, but brain edema rarely occurs in HHS. 1 , 9 At the time of admission, our patient's level of consciousness was low, which was due to high osmolality and brain edema (it was diagnosed clinically, and confirmed by brain CT scan). She also developed generalized convulsion; hence, she was intubated. Our goal was to gradually decrease serum osmolality and to administer fluids over a 72‐h period. Based on serum osmolality, urine out, and sodium level, we increased the amount of fluid. Moreover, neuroprotection was started for her (head of bed elevation 30‐degree, mannitol 20%, assuring adequate oxygenation by saturations >90%, avoiding hypercapnia by PaCO 2 between 34 and 38, and appropriate mean arterial pressure (MAP) to maintain adequate cerebral perfusion pressure in the range of 50–70, and aggressive fever control). 9
Deficit of potassium, magnesium, and phosphate in HHS is much greater than DKA. In our patient, on the 4th day of admission, serum phosphate decreased to 0.75 mg/dl.
In the previous studies, mortality rate has been reported up to 35% depending on the severity of dehydration, hyperosmolality, and patient's age. 5 However, by following the aforementioned therapeutic procedures, our patient was cured without any sequelae.
HHS is a rare complication of DM among pediatric patients, but with more complications and poorer outcome. Hence, pediatrician should be well aware of its presentations and signs for a timely diagnosis and treatment.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
AS designed the study and wrote the manuscript, in addition to data collection as well as submitting the manuscript. HI was the scientific consultant. AS and HI edited the manuscript collectively. Both authors discussed the results and contributed to the final manuscript.
ETHICAL APPROVAL
This study was approved by the local ethics committee of Shiraz University of Medical sciences with approval ID: IR.sums.med.rec.1398.134. Written informed consent was obtained from patient's parents and delivered to the ethics committee.
Written informed consent was obtained from the parents of the patient for publication purposes of this case report and any accompanying images. A copy of the written consent is available for review by the Chief Editor of the Journal.
ACKNOWLEDGEMENTS
The authors are grateful to the PICU nurses who were involved in the care of this patient, and they also would like to thank Dr. H. Argasi at the RT Publication for his invaluable assistance in editing this manuscript.
Saeed A, Ilkhanipoor H. A case report: First presentation of diabetes mellitus type 1 with severe hyperosmolar hyperglycemic state in a 35‐month‐old girl . Clin Case Rep . 2021; 9 :e04984. 10.1002/ccr3.4984 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or none‐profit sectors
DATA AVAILABILITY STATEMENT
- Open access
- Published: 27 May 2022
Breastfeeding, nutrition and type 1 diabetes: a case-control study in Izmir, Turkey
- İpek Çiçekli ORCID: orcid.org/0000-0003-4717-6145 1 &
- Raika Durusoy ORCID: orcid.org/0000-0003-1041-8462 2
International Breastfeeding Journal volume 17 , Article number: 42 ( 2022 ) Cite this article
6227 Accesses
10 Altmetric
Metrics details
The relationship between infant breastfeeding and type 1 diabetes mellitus (DM) is unclear but it has been suggested that there may be a link between many environmental factors, including dietary antigens affecting diabetes epidemiology.
The main objective of this study is to investigate nutritional risk factors, especially breastfeeding early in life that may be associated with the development of type 1 DM and to determine the relationship these factors have with the disease.
This research is a case-control study and was carried out in Ege University Children’s Hospital in İzmir, Turkey between 13 January 2020 and 5 March 2020. A total of 246 children aged between 4 and 14 years were included in the study. The case group consisted of patients diagnosed with type 1 DM followed-up by Ege University Children’s Hospital’s Endocrinology Unit and the control group included non-diabetic children attending the same hospital’s General Pediatric Outpatient Clinic. A structured questionnaire was created by the researchers after reviewing the literature related to nutritional and other risk factors for type 1 DM. The questionnaire was administered by interviewing the parents and it was related to the child, mother and family of the child. In this study, breastfeeding duration was defined as the total duration of breastfeeding and exclusive breastfeeding meant that the child received only breast milk from the mother.
The mean age at diagnosis was 6.30 ± 4.03 years for cases and 7.48 ± 2.56 years for controls. We found that each monthly increase in exclusive breastfeeding duration provided a 0.83-fold (95% CI 0.72, 0.96) decrease in the risk of type 1 DM. Introduction of cereals in the diet at the sixth month or earlier was associated with a 2.58-fold (95% CI 1.29, 5.16) increased risk.
Conclusions
Determining the contribution of exclusive breastfeeding to the disease is important in establishing preventive policies. A longer duration of exclusive breastfeeding may be an important role in preventing the disease. This free intervention that truly works will be cost-effective. Future studies are needed to clarify the role of both exclusive and non-exclusive breastfeeding on the development of type 1 DM.
Diabetes mellitus (DM) is a chronic metabolic disease characterized by hyperglycemia due to impairments in either insulin secretion and / or insulin effect [ 1 ]. As of today, 537 million people worldwide have diabetes [ 2 ]. This number is estimated to reach 643 million in 2030 and 783 million in 2045, which can be considered alarming levels [ 2 ].
Type 1 DM is characterized by insulin deficiency and hyperglycemia, usually starting in childhood, when the beta-cells of the pancreas are destroyed by autoimmune or non-autoimmune processes [ 2 ]. In individuals with genetic predisposition (human leukocyte antigen or HLA groups at risk), autoimmunity is triggered by the effect of environmental factors (viruses, toxins, emotional stress, others) and progressive beta-cell damage begins. Clinical symptoms of diabetes occur when beta-cell reserves are reduced by 80–90% [ 3 ].
It has been suggested that there are many environmental factors, including dietary antigens [ 4 , 5 , 6 ], as well as genetic risk factors [ 7 , 8 , 9 , 10 , 11 ] that affect the epidemiology of type 1 DM [ 12 ]. Although not all genotypes with risk have yet been identified, only about 10–15% of individuals at genetic risk develop type 1 DM [ 5 ]. In studies conducted on migrants, it has been shown that the incidence of type 1 DM increases in those who migrate from a region where the incidence of type 1 DM is low to a region with high incidence, and the effect of environmental conditions has been emphasized [ 13 ]. These data were found to be consistent with the results of studies finding that environmental triggers increase and accelerate the development of clinical type 1 DM despite lower genetic predisposition [ 13 ].
Some nutritional factors contribute to the development of the disease. Studies in 40 countries worldwide have shown that dietary patterns may impact the development of type 1 DM [ 14 ]. Vitamin D, another nutritional factor, may have a protective effect on glycemic control in patients with type 1 DM [ 15 ] and according to a birth cohort study, the provision of vitamin D supplementation for infants early in life could help to reduce the risk of the disease [ 16 ]. The introduction of cow’s milk-based infant formulas in the first three postnatal months was found to be associated with an increase in pancreatic beta-cell auto-antibodies [ 17 ]. However, another study had shown that cow’s milk did not play an important role in the development of type 1 DM [ 18 ].
Although many studies have been performed to investigate the role of nutrition in pregnancy and early in life on type 1 DM, the results have been inconsistent. Breastfeeding [ 19 ], probiotic supplementation [ 20 ], vitamin C, and zinc supplementation [ 21 ] have been shown as possible protective factors against type 1 DM whereas early exposure to eggs, gluten [ 22 , 23 ] and vegetables [ 24 ] might increase the risk.
Studies with school-age children have shown that diabetic children are significantly more prone to stress and depression compared to non-diabetic children [ 25 ]. Beyond the psychological and somatic effects of the disease on the individuals, diabetic individuals also encounter socio-economic consequences affecting their families and entire societies [ 26 ]. Frequent co-morbidities further increase negative socioeconomic consequences, especially in low- and middle-income countries [ 26 ].
According to the Social Security Institution’s data in Turkey, the costs of diabetes and its complications amount to approximately 23% of the total health expenditure [ 27 ]. In addition, indirect costs such as the loss of productivity of diabetics, the persons caring for the patient and their family are not included in these cost estimates. Therefore the cost does not reflect the psychosocial effects of the losses of quality-adjusted life years. Knowledge of modifiable environmental risk factors in type 1 DM can assist authorities in planning and implementing preventive policies to reduce the burden of the disease. It is as yet uncertain how and which nutritional or other environmental factors are important in the development of type 1 DM. Moreover, epigenetic mechanisms are not clearly defined.
The main objective of this study is to investigate potential nutritional risk factors, especially breastfeeding early in life, that may be associated with the development of type 1 DM and to determine the relationship of these factors with the disease, independent of other established risk factors.
Participants
A case-control study was carried out at Ege University Children’s Hospital, İzmir City, Turkey, over a period of two months from January to March 2020.
A minimum sample size of 105 cases and 105 controls with a total of 210 participants was calculated with G-Power using the t-test group, with an effect size of 0.5, an error margin of 0.05, and a power of 95%. About 20% more sample size was added to account for possible non-response and a total of 246 children (120 cases and 126 controls) were included in the study.
The study data were collected at Ege University Faculty of Medicine Children’s Hospital in Bornova, Izmir between 13 January 2020 and 5 March 2020. Children and their parents who attended the general pediatrics and endocrinology / metabolic diseases outpatient clinics of the hospital and who met the study criteria were examined. The case group consisted of 120 children in the age group of 4–14 years who were diagnosed with type 1 DM based on World Health Organization and International Diabetes Federation guidelines [ 28 ] and who were being followed-up at Ege University Children’s Hospital Endocrinology / metabolic diseases outpatient clinic.
The diabetes outpatient clinic is held once a week (on Thursdays) and on the first Monday of every month. The mean number of diabetic patients attending the research was 15 patients per day. The control group comprised 126 non-diabetic children selected from the general pediatric outpatient clinic of the same hospital. A questionnaire was applied face-to-face to the parents of the children. All questions in the study were asked to the parents and separately written informed consent was obtained from children and their parents. In addition, the files of the case group were examined and the date of diagnosis, height, body weight and HbA1c levels at the time of diagnosis were collected as data.
Children who were followed up in the Endocrine and Metabolic Diseases Outpatient Clinic, diagnosed with type 1 DM and aged between 4 and 14 years were included in the case group. Children who attended the General Pediatrics Outpatient Clinic, were not diagnosed with type 1 DM, and aged 4–14 years were included in the control group. Those who did not want to share their information and could not remember answers to the study questions were excluded. The response rates were 96 and 91% among cases and controls, respectively, for all eligible cases and controls attending the hospital.
Questionnaires
A structured questionnaire was created by the researchers after reviewing the literature related to nutritional and other risk factors for type 1 DM [ 21 , 29 , 30 , 31 , 32 , 33 , 34 ]. The questionnaire was administered by interviewing the parents and its content was related to the child, mother and family of the child. For children: anthropometric data, breastfeeding duration, infant formula consumption, the introduction of some foods into the diet, infections, supplementations (vitamin D and probiotic) early in life and physical activity were questioned; for mothers, anthropometric data and history during pregnancy; for family, socio-demographic characteristics such as education, whether the child lived with parents, and family history were asked. In addition, the case group was examined about the age at diagnosis of the disease, the HbA1c level and the percentiles at diagnosis.
In this study, breastfeeding duration was defined as the total duration of breastfeeding and exclusive breastfeeding meant that the child received only breast milk (no other liquids or solids given, not even water with the exception of oral rehydration solution, or drops / syrups of vitamins, minerals or medicines) from the mother [ 35 ].
The percentiles were calculated based on the percentile values table of Neyzi et al. [ 36 ]. Parents’ body mass index (BMI) was classified according to the World Health Organization’s obesity scale [ 37 ]. Finally, high-intensity physical activity was defined as “physical activities that increase the maximum heart rate by 70 − 85%” [ 38 ]. Examples of physical activities were given (running, basketball, football, tennis, swimming, skipping rope) by the researcher.
Statistical analysis
The data were analyzed by using SPSS software. The quality of the data had been checked prior to analysis. Descriptive variables of cases and controls were compared with Student t-tests (continuous variables), Mann Whitney U tests (non-parametric) and chi-square tests (categorical variables). In order to reveal the relationship between significant parameters and the development of type 1 DM independently from other factors, age and sex-adjusted logistic regression analysis were performed. Since the difference in mean ages of the two groups was found to be significant (both age of enrolment in the study and age at diagnosis type 1 DM), other variables were evaluated adjusting for age and gender.
General pediatric outpatient clinic admissions are due to newly developing acute conditions and 85–90% are first visits to the hospital. Ten to 15 % are invited for follow-up one month later, so the follow-up is also at the same age. If they also have a chronic condition, they are referred to pediatric specialization clinics and start follow-up in those clinics.
Among the cases, six were diagnosed with type 1 DM at zero years, three of whom were excluded from the multivariate analysis since they were diagnosed in their first month of life, so the diagnosis would be before the environmental exposures could happen. The remaining three children were diagnosed at 10, 11 and 11 months, thus they were kept in the analysis since they could be exposed to potential nutritional risk factors in question.
Sex and age-adjusted multivariable logistic analysis, adjusted odds ratios (aORs) and 95% confidence intervals (95% CIs) were used to identify possible risk factors of the disease. In all analyses, p < 0.05 was considered statistically significant. The dependent variable was having type 1 DM. Maternal factors, family history, family characteristics, nutritional characteristics early in life were the independent variables.
Population Attributable Risk (PAR) and Population Attributable Risk Percent (PAR %) were calculated to estimate the proportion of cases for whom the disease is attributable to exclusive breastfeeding and to estimate the excess rate of type 1 DM in the study population of both exposed and non-exposed children that is attributable to being non-breastfed exclusively up to the first six months. This measure was calculated as [ 39 ]:
Characteristics of children
A total of 246 children were included in the study, with 120 cases and 126 controls. The mean age of the case and control groups was 10.43 ± 3.31 and 7.48 ± 2.56 years, respectively ( p < 0.05). The cases’ mean age at diagnosis was 6.30 ± 4.03 years and was found to be significantly lower than the control group. The mean duration of their disease was 4.2 ± 3 .85 years. The mean height percentile was higher in controls (means 45.66 ± 31.16 and 58.00 ± 31.88, p = 0.003) and the mean BMI percentile was higher in the case group (means 55.20 ± 29.86 and 40.32 ± 35.02, p < 0.001). A significant difference was found in the family history of type 1 DM. There was a type 1 DM history in 10.7% of the case group and 0.8% in the control group ( p = 0.001). No significant difference was found in the child’s living status with parents and parents’ education level. However, a significant difference was found in physical activity levels ( p = 0.014). There was no difference between the duration of vitamin D use. In both groups, no infant was supplemented with probiotics in the first year postpartum. The controls’ rate of living in urban areas was found to be significantly higher (Table 1 ).
Maternal characteristics
The mean birth interval was higher in the case group. A significant difference was found in the birth intervals with univariate analysis ( p = 0.036) but not in multivariate analysis. Those with a birth interval of more than six years constituted 20.7% of the cases and 8.0% of controls (Table 2 ). In the case group, no mother was supplemented with probiotics during pregnancy and 98.4% of the control group were not supplemented with probiotics during pregnancy.
Nutritional profiles of children
The mean duration of exclusive breastfeeding was higher in the control group ( p = 0.009). In the case group, the rate of exclusive breastfeeding for less than one month was 47.8, and 30.6% in the controls (Table 3 ). This difference was statistically significant ( p = 0.037). No statistically significant differences were found between colostrum consumption, total breastfeeding duration, infant formula consumption and formula preferences.
No statistically significant difference was observed between which month the cow’s milk, eggs, fruits, vegetables, and berry fruits were introduced. However the introduction of cereals was statistically significant and the cases’ introduction to them was earlier ( p = 0.008). For the case group 5.5% were introduced to cereals before the sixth month as compared to 3.2% of controls, while 44.2% of controls were introduced to cereals after the eighth month, compared to 24.7% of cases (Table 4 ).
Multivariate analysis
According to non-parametric correlation analyses, exclusive breastfeeding duration and total breastfeeding duration were not found to be associated with age when type 1 DM was diagnosed. The birth interval was found to be significant in the age and sex-adjusted regression analysis. In addition, regardless of age and gender, it was observ ed. that the risk of type 1 DM decreased 0.85 ( p = 0.007; 95% CI0.76, 0.96) times with each monthly increase in the duration of exclusive breastfeeding (Fig. 1 ). Having a birth interval of more than six years increased the risk of the disease by 2.79 ( p = 0.018; 95% CI 1.19, 6.54) times.
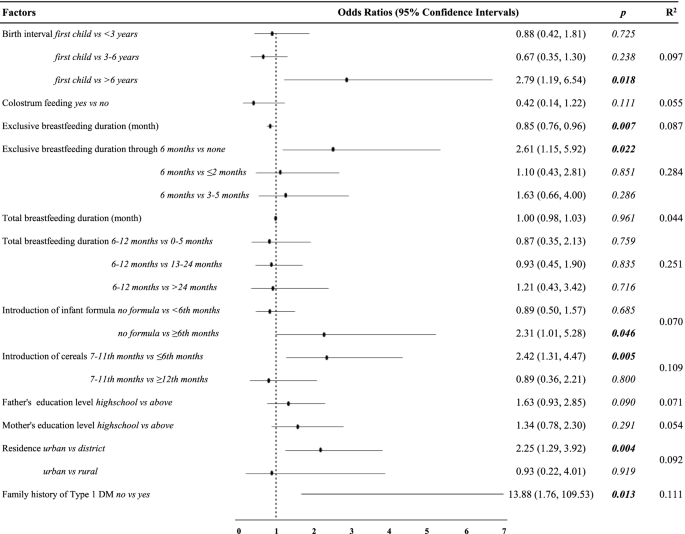
Sex and age adjusted (the cases’ age at diagnosis) logistic regression analysis of risk factors independent of other risk factors
According to results of the multivariate logistic regression, longer exclusive breastfeeding duration, living in a rural area and not consuming infant formula were identified as protective factors. Although there was no significant difference found in type 1 DM risk with introduction to cereals at 12 months and after, it was found that the introduction to cereals at the sixth month and earliere increased the risk of type 1 DM by 2.58 ( p = 0.008; 95% CI 1.29, 5.16) times compared to between months7–11, independent of other risk factors. Similarly, infant formula consumption after the sixth month was associated with an increased risk of type 1 DM compared to no infant formula consumption (Table 5 ).
Sensitivity analyses
The potential impacts on our results of age at which the cases developed DM, (whether including or excluding data for the three children who were diagnosed at the first month after birth) and with data missing for the father’s education level variable, was assessed using multiple imputations, as described in the Supplementary Data . Among the cases, six of them were diagnosed with type 1 DM in the first year of life, three of whom were excluded from the multivariate analysis since they were diagnosed in their first month of life, so the diagnosis would have occurred before the environmental exposures could happen. The remaining one child was diagnosed at ten months and two children at 11 months, thus they were kept in the analysis since they could have been exposed to the potential nutritional risk factors in question.
The main analyses were repeated after adjusting for age and the father’s education level. Multiple imputations changed some of the conclusions based on the research sample as attached (Supplementary Table 1 ). The missing data on the father’s education level in the study was assessed using multiple imputations and this did not change the conclusions. So the model excluding the father’s education level and cases that developed type 1 DM before the first month was used.
PAR was calculated as 0.111 in the study and PAR% was calculated as 38.3% .
Elimination of preventable environmental risk factors associated with type 1 DM is an important step in the prevention of the disease. However, it has not been precisely explained which factors play a key role and when and in which situations the factors should be eliminated [ 22 ]. In this research we have explored possible preventable environmental triggers and determinants, especially breastfeeding early in life.
We found that each monthly increase in the duration of exclusive breastfeeding but not total breastfeeding provides a reduction in type 1 DM risk. However, introducing the cereals before the sixth month was found to be an important risk factor. The birth interval which was significant in univariate analyzes, lost its significance in multivariate analysis.
- Breastfeeding
The effect of breast milk, the first food of the newborn, on type 1 DM is a controversial issue. There are many studies in the literature that show no effect [ 40 ], a protective effect [ 19 ] and an effect [ 21 ]. It has been suggested that the protective effect of breastmilk is through reducing neonatal intestinal permeability [ 41 ]. The World Health Organization recommends feeding exclusively breast milk in the first six months of life and breastmilk up to the age of two, because feeding children with exclusively breast milk for the first six months after birth prevents diarrhea, respiratory diseases and provides all the nutrients and fluids the infant needs for optimal growth and development [ 42 ]. For participants in our study, it was observed that the rates of those who did not receive breast milk at all or those who were exclusively breastfed for less than a month were quite high. It has been observed that the accomplishment rate of the World Health Organization target for six months exclusive breastfeeding is low.
According to the Turkey Demographic and Health Survey data 2018, approximately two in five children were exclusively breastfed up to six months old and the proportion of children who are exclusively breastfed decreases with age; from 59% among 0–1 month-old infants to 14% among 4–5 months old infants [ 43 ]. On the other hand, the National Immunization Survey results indicated that only one in four children was breastfed exclusively through six months in the U.S. [ 44 ]. In our study, only the median month of breastfeeding was close to the Turkey Demographic and Health Survey data. The exclusive breast milk receiving rates through six months were found to be lower than the worldwide, National Immunization Survey and Turkey Demographic and Health Survey data in both the case and control groups. This was not surprising because our sample was quite low compared to the aforementioned samples, and the data mentioned reflected a population of children younger than two years old in 2018. So the mean age of the children in our study was higher. This result may be different in studies to be conducted with a larger population and adjusted for age. There are large differences in breastfeeding rates between regions, between and within countries. But unfortunately, these rates are insufficient both in the world and in Turkey. We can estimate that 38.3% of type 1 DM cases would be avoided by an increase in the proportion of infants exclusively breastfed to six months. Keep in mind that almost two in five infants who are not breastfed exclusively for the first six months will have type 1 DM so any intervention that can promote breastfeeding may have a big impact in preventing the disease.
In the study of Çarkçı and Altuğ (2020), conducted in the same city as this study (İzmir) the rate of children with type 1 DM who received exclusive breast milk up to the first six months was found to be more than four times compared to our study [ 45 ]. While asking the duration of exclusive breastfeeding, the definition of exclusive breastfeeding was explained as “the total time in which the baby takes only breast milk, and no other liquid (including water) or solids other than oral rehydration solution or vitamins, minerals or drugs/syrups are given” in this study. While making a statement, after the parents answered, “Have you ever given water during this period?” was asked again to be sure. In this process, there were parents who changed their answers after the second question. Therefore, different results may have been obtained in studies where this distinction was not made clear.
There are many studies on the relationship between breastfeeding and type 1 DM. Holmberg et al. (2007) found that the duration of total breastfeeding for less than four months is a risk factor for the development of beta-cell autoimmunity in children under five years old. The same study reported that the duration of exclusive breastfeeding for less than four months increased the risk of developing beta-cell autoantibodies two times [ 17 ]. In another study, it was shown that the risk of type 1 DM in childhood can be reduced by 15%, even by breastfeeding exclusively in the early weeks of life. However, the observed relationship between exclusive breastfeeding and type 1 DM could not be explained independently of certain risk factors for DM such as gestational DM, birth weight, gestational age, maternal age, birth order and mode of delivery [ 19 ]. However in a series of prospective and birth cohort studies investigating the relationship between breastfeeding and the development of islet autoimmunity, no effect of breastfeeding has been reported [ 46 , 47 ]. Similarly, a series of prospective studies investigating the relationship between breastfeeding and the development of type 1 DM reported that breastfeeding had no effect [ 48 , 49 ].
We found that longer exclusive breastfeeding duration was a significant protective factor against type 1 DM but the same effect was not observed with total breastfeeding duration. In some studies, exclusive breastfeeding and total breastfeeding duration were not compared and the duration of total or any breastfeeding was researched. Therefore the differences in studies’ results can be attributed to their methods.
In addition to distinguishing between exclusive breastfeeding and total breastfeeding, there are also differences between studies in defining exclusive breastfeeding. For instance, in two Large Scandinavian Birth Cohorts, breastfed infants were found to be at doubled risk of type 1 DM compared to infants who did not receive breast milk at all but no evidence indicated that longer duration of breastfeeding was associated with a reduced risk of the disease [ 50 ].
Similarly, in two large cohort studies, breastfeeding duration was not associated with type 1 DM [ 51 , 52 ]. Infants classified as exclusively breastfed were allowed water-based drinks in the aforementioned study and duration of exclusive breastfeeding was not taken into account.
As can be seen, many studies only look at total breastfeeding duration without making any distinction between exclusive breastfeeding duration and total breastfeeding duration. In addition, striking differences in the breastfeeding practices of governments and health authorities may be a confounding factor in the results of the studies. Positive social norms that support and encourage breastfeeding, including in public spaces, encourage mothers to breastfeed [ 53 ]. As observed in our study, the duration of exclusive breastfeeding of children after birth is quite low and it has been observed that parents do not attach sufficient importance to the period of exclusive breastfeeding.
Support from trained counselors and peers, including mothers and other family members, is as important as postpartum health care in maintaining breastfeeding in communities. The support of men, spouses and partners should not be ignored in this process [ 53 ]. In studies on breastfeeding, the mother has always been at the center and studies on the role of fathers / partners are insufficient. Tohotoa et al. highlighted the importance of the role of fathers in encouraging and supporting a successful breastfeeding process [ 54 ]. Moreover, paternal practical, physical and emotional support could make a difference [ 54 ].
When challenges experienced by mothers are shared with their partners, babies might have a better chance of receiving exclusive breast milk for the recommended six months and could keep going on breastfeeding for up to two years. In this way, the early introduction of complementary foods, especially cereals, which we found a significant risk factor for type 1 DM in our study, could be prevented.
Cow’s milk and infant formula consumption
Early exposure to cow’s milk proteins has been studied in terms of beta-cell autoimmunity and the risks of clinical disease development [ 55 ]. Early introduction of cow’s milk proteins into the diet may trigger inflammation of the intestinal mucosa and increase intestinal permeability [ 56 ]. The introduction of infant formula reflects the total duration of the exclusive breastfeeding [ 31 ]. Therefore, it should be considered together with the duration of exclusive breastfeeding. These may have led to contradictory results [ 31 ].
Some studies have shown that early exposure to cow’s milk proteins increases the risk of beta-cell autoimmunity [ 57 ] and type 1 DM [ 58 ] while others found no relationship between type 1 DM and cow’s milk proteins [ 31 , 59 ]. We also did not find an association with the timing of cow’s milk introduction. It has been observed that consumption of infant formula at six months and later increased the risk of type 1 DM in this research. However, while the risk of type 1 DM was expected to increase with the consumption of infant formula at six months and earlier compared to those who did not consume it, a statistically significant change was not observed.
This result may be explained in three ways: First, there may be a bias in choosing the control group from the same tertiary care and university hospitals with type 1 DM patients. Considering the socioeconomic status of children attending a university hospital, infant formula may have been introduced earlier than the general community and might not represent the healthy control group clearly. Second, there may have been a response bias. Third, since the mean age of children with type 1 DM is significantly higher, parents may have recall bias. Although it is easy to identify potential sources of bias, it is not possible to predict the true impact of these biases on results.
Introduction to cereals
Gluten, a protein found in barley, wheat and rye has been hypothesized to be one of the nutritional risk factors related to the development of type 1 DM [ 60 ]. A study in non-obese diabetic rats concluded that the intra-epithelial infiltration of T cells, the incidence of autoimmune type 1 DM and enteropathies decreased with a gluten-free diet compared to the controls [ 61 ]. Introduction of gluten before four months of age was associated with an increased risk of type 1 DM in another study [ 62 ]. These results were explained by the hypothesis that the gluten-free diet may prevent gliadin peptides from crossing the intestinal barrier by reducing intestinal permeability, thus preventing the development of pancreatic autoimmunity [ 63 ]. Our study supports these arguments since introducing cereals before the sixth month was found to be an important risk factor.
However in our study, the cereals were not questioned for their gluten content, they were questioned overall, but wheat production and consumption ranks in the first place among cereals in Turkey [ 64 ]. So wheat-containing cereals (including gluten) are expected to be added into the diet of infants in the transition to complementary foods at first. Nevertheless, it could not be confirmed specifically that gluten exposure was earlier in cases, but it was found that cereal introduction prior to the sixth month was associated with an increased risk of type 1 DM.
Limitations
We have many limitations in the study. As in our study, case-control studies always have the potential for bias. It is not easy to collect accurate and unbiased data on past exposures. Therefore case-control studies are prone to some sources of bias like recall bias or the control group’s selection from the hospital. Many of the established risk factors were questioned, in order to overcome confounding. However, the gestation week was not questioned at birth, so it could not be evaluated whether the birth weight was normal for gestational age. It was questioned whether they drank water during exclusive breastfeeding, but we did not collect data on when they started to consume water. Therefore this variable maybe could provide a better comparison for exclusive breastfeeding duration in future studies. In addition, the vaccination status of the children was not asked and abortion was not researched while questioning the birth interval and birth order so they may be confounding factors. Since infections in the first three years were questioned by anamnesis, their bacterial / viral status could not be determined and their relationship with enteroviruses could not be investigated.
Longer exclusive breastfeeding duration may prevent the early introduction of certain nutrients in the diet. Determining the contribution of exclusive breastfeeding and its interactions with protective factors to the disease is important in establishing preventive policies. Breastfeeding is cost-effective and may be a free intervention for the prevention of type 1 DM. Support from partners is a key factor in maintaining breastfeeding in communities. Considering the limitations of the study, systematic reviews with meta-analysis are needed in determining the role of both exclusive and non-exclusive breastfeeding on the development of type 1 DM.
Availability of data and materials
The dataset could be obtained from the corresponding author upon reasonable request.
Abbreviations
Human leukocyte antigen
Population attributable risk
Population attributable risk percent
National Diabetes Consensus Group. TURKDIAB Diabetes Diagnosis and Treatment Guideline 2017. 7th edition. İstanbul; 2017. http://www.turkdiab.org/admin/PICS/webfiles/Diyabet_tani_ve_tedavi__kitabi.pdf . Accessed 25 May 2021.
International diabetes federation. Diabetes atlas 2021. 10th ed. Brussels: International Diabetes Federation; 2021. https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf .
Turkey Endocrinology and Metabolism Society. Endocrinology and Metabolism Association Guideline 2019. 14th edition. Ankara; 2019. https://temd.org.tr/admin/uploads/tbl_kilavuz/20200625154506-2020tbl_kilavuz86bf012d90.pdf . Accessed 29 Apr 2020.
Åkerblom HK, Vaarala O, Hyöty H, Ilonen J, Knip M. Environmental factors in the etiology of type 1 diabetes. Am J Med Genet Semin Med Genet. 2002;115:18–29.
Article Google Scholar
Esposito S, Toni G, Tascini G, Santi E, Berioli MG, Principi N. Environmental factors associated with type 1 diabetes. Front Endocrinol. 2019;10:592. https://doi.org/10.3389/fendo.2019.00592 .
D’Angeli MA, Merzon E, Valbuena LF, Tirschwell D, Paris CA, Mueller BA. Environmental factors associated with childhood-onset type 1 diabetes mellitus: an exploration of the hygiene and overload hypotheses. Arch Pediatr Adolesc Med. 2010;164:732–8. https://doi.org/10.1001/archpediatrics.2010.115 .
Article PubMed PubMed Central Google Scholar
Redondo MJ, Concannon P. Genetics of type 1 diabetes comes of age. Diabetes Care. 2020;43:16–8. https://doi.org/10.2337/dci19-0049 .
Article PubMed Google Scholar
DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391:2449–62. https://doi.org/10.1016/S0140-6736(18)31320-5 .
Pociot F, Akolkar B, Concannon P, Erlich HA, Julier C, Morahan G, et al. Genetics of type 1 diabetes: What’s next? Diabetes. 2010;59:1561–71. https://doi.org/10.2337/db10-0076 .
Article CAS PubMed PubMed Central Google Scholar
Howson JMM, Rosinger S, Smyth DJ, Boehm BO, Aldinger G, Aufschild J, et al. Genetic analysis of adult-onset autoimmune diabetes. Diabetes. 2011;60:2645–53. https://doi.org/10.2337/db11-0364 .
Soltész G. Diabetes in the young: A paediatric and epidemiological perspective. Diabetologia. 2003;46:447–54. https://doi.org/10.1007/s00125-003-1101-0 .
Piescik-Lech M, Chmielewska A, Shamir R, Szajewska H. Systematic review: early infant feeding and the risk of type 1 diabetes. J Pediatr Gastroenterol Nutr. 2017;64:454–9.
Bodansky HJ, Staines A, Stephenson C, Haigh D, Cartwrigth R. Evidence for an environmental effect in the aetiology of insulin dependent diabetes in a transmigratory population. BMJ. 1992;304:1020–2. https://doi.org/10.1136/BMJ.304.6833.1020 .
Muntoni S, Cocco P, Aru G, Cucca F, Muntoni S. Nutritional factors and worldwide incidence of childhood type 1 diabetes. Am J Clin Nutr. 2000;71:1525–9.
Article CAS Google Scholar
Aljabri KS, Bokhari SA, Khan MJ. Glycemic changes after vitamin D supplementation in patients with type 1 diabetes mellitus and vitamin D deficiency. Ann Saudi Med. 2010;30:454. https://doi.org/10.4103/0256-4947.72265 .
Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet. 2001;358:1500–3.
Holmberg H, Whalberg J, Vaarala O, Ludvigsson J. Short duration of breast-feeding as a risk-factor for β-cell autoantibodies in 5-year-old children from the general population. Br J Nutr. 2007;97:111–6.
Knip M, Åkerblom HK, Altaji E, Becker D, Bruining J, Castano L, et al. Effect of hydrolyzed infant formula vs conventional formula on risk of type 1 diabetes the TRIGR randomized clinical trial. JAMA. 2018;319:38–48. https://doi.org/10.1001/jama.2017.19826 .
Cardwell CR, Stene LC, Ludvigsson J, Rosenbauer J, Cinek O, Svensson J, et al. Breast-feeding and childhood-onset type 1 diabetes: A pooled analysis of individual participant data from 43 observational studies. Diabetes Care. 2012;35:2215–25. https://doi.org/10.2337/dc12-0438 .
Xiao L, Van’t Land B, Engen PA, Naqib A, Green SJ, Nato A, et al. Human milk oligosaccharides protect against the development of autoimmune diabetes in NOD-mice. Sci Rep. 2018;8:1–15.
Google Scholar
Virtanen SM, Knip M. Nutritional risk predictors of β cell autoimmunity and type 1 diabetes at a young age. Am J Clin Nutr. 2003;78:1053–67.
Esposito S, Toni G, Tascini G, Santi E, Berioli MG, Principi N. Environmental factors associated with type 1 diabetes. Front Endocrinol. 2019;10:592.
Norris JM, Barriga K, Klingensmith G, Hoffman M, Eisenbarth GS, Erlich HA, et al. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. J Am Med Assoc. 2003;290:1713–20. https://doi.org/10.1001/jama.290.13.1713 .
Virtanen SM, Takkinen HM, Nevalainen J, Kronberg-Kippilä C, Salmenhaara M, Uusitalo L, et al. Early introduction of root vegetables in infancy associated with advanced ß-cell autoimmunity in young children with human leukocyte antigen-conferred susceptibility to type 1 diabetes. Diabet Med. 2011;28:965–71. https://doi.org/10.1111/j.1464-5491.2011.03294.x .
Article CAS PubMed Google Scholar
Streisand R, Monaghan M. Young children with type 1 diabetes: challenges, research, and future directions. Curr Diabetes Rep. 2014;14:520.
World Health Organization. Classification of Diabetes Mellitus 2019. Geneva; 2019. https://www.who.int/publications/i/item/classification-of-diabetes-mellitus . Accessed 11 Apr 2020.
Social Security Institution of Turkey. Diabetes from the Social Security Institution’s Perspective. Ankara; 2014. https://veri.sgk.gov.tr . Accessed 11 Apr 2020.
World Health Organization; International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia Report of a WHO/IDF Consultation. Geneva; 2006. https://apps.who.int/iris/bitstream/handle/10665/43588/9241594934_eng.pdf?sequence=1&isAllowed=y . Accessed 1 Jan 2021.
Awadalla NJ, Hegazy AA, El-Salam MA, Elhady M. Environmental factors associated with type 1 diabetes development: A case control study in Egypt. Int J Environ Res Public Health. 2017;14:615. https://doi.org/10.3390/ijerph14060615 .
Article PubMed Central Google Scholar
Muntoni S, Mereu R, Atzori L, Mereu A, Galassi S, Corda S, et al. High meat consumption is associated with type 1 diabetes mellitus in a Sardinian case-control study. Acta Diabetol. 2013;50:713–9.
Knip M, Simell O. Environmental triggers of type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2:1–15.
Knip M, Virtanen SM, Becker D, Dupre J, Krischer JP, A HK. Early feeding and risk of type 1 diabetes: experiences from the trial to reduce insulin-dependent diabetes mellitus in the genetically at risk (TRIGR) 1-6. Am J Clin Nutr. 2011;94:1814S–20.
Nygren M, Carstensen J, Koch F, Ludvigsson J, Frostell A. Experience of a serious life event increases the risk for childhood type 1 diabetes : the ABIS population-based prospective cohort study. Diabetologia. 2015;58:1188–97.
Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387:2340–8. https://doi.org/10.1016/S0140-6736(16)30507-4 .
World Health Organization. Exclusive breastfeeding for optimal growth, development and health of infants. 2019. https://www.who.int/elena/titles/exclusive_breastfeeding/en/ . Accessed 2 Jan 2021.
Neyzi O, Günöz H, Furman A, Bundak R, Gökçay G, Darendeliler F, et al. Body weight, height, head circumference and body mass index reference values in Turkish children. Cocuk Sagligi ve Hastalikari Dergisi. 2008;51(1):1.
World Health Organization. Health Topics: Obesity. https://www.who.int/health-topics/obesity#tab=tab_1 . Accessed 2 Jan 2021.
Republic of Turkey Ministry of Health, General Directorate of Public Health. Turkey Physical Activity Guidelines. 2nd edition. Ankara; 2014. https://hsgm.saglik.gov.tr/depo/birimler/saglikli-beslenme-hareketli-hayat-db/Fiziksel_Aktivite_Rehberi/Turkiye_Fiziksel_Aktivite_Rehberi.pdf .
Hennekens CH, Buring JE. Measures of Disease Frequency and Association. In: Mayrent SL, editor. Epidemiology in Medicine. 1st ed. Boston: Little Brown; 2012. p. 96.
Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA. 2003;290:1721–8. https://doi.org/10.1001/JAMA.290.13.1721 .
Catassi C, Bonucci A, Coppa GV, Carlucci A, Giorgi PL. Intestinal permeability changes during the first month: effect of natural versus artificial feeding. J Pediatr Gastroenterol Nutr. 1995;21:383–6. https://doi.org/10.1097/00005176-199511000-00003 .
World Health Organization. Health Topics: Breastfeeding. https://www.who.int/health-topics/breastfeeding#tab=tab_1 . Accessed 25 Jan 2022.
Hacettepe University Institute of Population Studies. Turkey Demographic and Health Survey 2018 Main Report. Ankara/Turkey; 2019. www.hips.hacettepe.edu.tr . Accessed 9 Nov 2021.
Centers for Disease Control and Prevention. Results: Breastfeeding Rates. https://www.cdc.gov/breastfeeding/data/nis_data/results.html . Accessed 11 Nov 2021.
Çarkçı NŞ, Altuğ ÖS. Studying the epidemiologic characteristics of children with type 1 diabetes followed in İzmir. J Educ Res Nurs. 2020;17:24–31.
Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. J Am Med Assoc. 2003;290:1721–8. https://doi.org/10.1001/jama.290.13.1721 .
Virtanen SM, Kenward MG, Erkkola M, Kautiainen S, Kronberg-Kippilä C, Hakulinen T, et al. Age at introduction of new foods and advanced beta cell autoimmunity in young children with HLA-conferred susceptibility to type 1 diabetes. Diabetologia. 2006;49:1512–21. https://doi.org/10.1007/s00125-006-0236-1 .
Viner RM, Hindmarsh PC, Taylor B, Cole TJ. Childhood body mass index (BMI), breastfeeding and risk of type 1 diabetes: findings from a longitudinal national birth cohort. Diabet Med. 2008;25:1056–61. https://doi.org/10.1111/J.1464-5491.2008.02525.X .
Frederiksen B, Kroehl M, Lamb MM, Seifert J, Barriga K, Eisenbarth GS, et al. Infant exposures and development of type 1 diabetes mellitus: the diabetes autoimmunity study in the young (DAISY). JAMA Pediatr. 2013;167:808–15. https://doi.org/10.1001/JAMAPEDIATRICS.2013.317 .
Lund-Blix NA, Sander SD, Størdal K, Nybo Andersen AM, Rønningen KS, Joner G, et al. Infant feeding and risk of type 1 diabetes in two large Scandinavian birth cohorts. Diabetes Care. 2017;40:920–7. https://doi.org/10.2337/DC17-0016/-/DC1 .
Ponsonby AL, Pezic A, Cochrane J, Cameron FJ, Pascoe M, Kemp A, et al. Infant anthropometry, early life infection, and subsequent risk of type 1 diabetes mellitus: a prospective birth cohort study. Pediatr Diabetes. 2011;12:313–21. https://doi.org/10.1111/J.1399-5448.2010.00693.X .
Savilahti E, Saarinen KM. Early infant feeding and type 1 diabetes. Eur J Nutr. 2009;48:243–9. https://doi.org/10.1007/S00394-009-0008-Z .
UNICEF. Breastfeeding: A Mother’s Gift, for Every Child. New York; 2018. https://www.unicef.org/media/48046/file/UNICEF_Breastfeeding_A_Mothers_Gift_for_Every_Child.pdf . Accessed 2 Feb 2022.
Tohotoa J, Maycock B, Hauck YL, Howat P, Burns S, Binns CW. Dads make a difference: an exploratory study of paternal support for breastfeeding in Perth, Western Australia. Int Breastfeed J. 2009;4:15. https://doi.org/10.1186/1746-4358-4-15 .
Šipetić S, Vlajinac H, Kocev N, Bjekić M, Sajic S. Early infant diet and risk of type 1 diabetes mellitus in Belgrade children. Nutrition. 2005;21:474–9. https://doi.org/10.1016/J.NUT.2004.07.014 .
Knip M, Virtanen SM, Åkerblom HK. Infant feeding and the risk of type 1 diabetes. Am J Clin Nutr. 2010;91:1506S. https://doi.org/10.3945/AJCN.2010.28701C .
Virtanen SM, Nevalainen J, Kronberg-Kippilä C, Ahonen S, Tapanainen H, Uusitalo L, et al. Food consumption and advanced β cell autoimmunity in young children with HLA-conferred susceptibility to type 1 diabetes: a nested case-control design. Am J Clin Nutr. 2012;95:471–8. https://doi.org/10.3945/AJCN.111.018879 .
Wahlberg J, Vaarala O, Ludvigsson J. Dietary risk factors for the emergence of type 1 diabetes-related autoantibodies in 21/2 year-old Swedish children. Br J Nutr. 2006;95:603–8. https://doi.org/10.1079/BJN20051676 .
Patterson C. Rapid early growth is associated with increased risk of childhood type 1 diabetes in various European populations. Diabetes Care. 2002;25:1755–60. https://doi.org/10.2337/DIACARE.25.10.1755 .
Barbeau WE, Bassaganya-Riera J, Hontecillas R. Putting the pieces of the puzzle together - a series of hypotheses on the etiology and pathogenesis of type 1 diabetes. Med Hypotheses. 2007;68:607–19. https://doi.org/10.1016/J.MEHY.2006.07.052 .
Maurano F, Mazzarella G, Luongo D, Stefanile R, D’Arienzo R, Rossi M, et al. Small intestinal enteropathy in non-obese diabetic mice fed a diet containing wheat. Diabetologia. 2005;48:931–7. https://doi.org/10.1007/S00125-005-1718-2 .
Lund-Blix NA, Dong F, Mårild K, Seifert J, Barón AE, Waugh KC, et al. Gluten intake and risk of islet autoimmunity and progression to type 1 diabetes in children at increased risk of the disease: the diabetes autoimmunity study in the young (DAISY). Diabetes Care. 2019;42:789. https://doi.org/10.2337/DC18-2315 .
Haupt-Jorgensen M, Holm LJ, Josefsen K, Buschard K. Possible prevention of diabetes with a gluten-free diet. Nutrients. 2018;10. https://doi.org/10.3390/NU10111746 .
Republic of Turkey Ministry of Food Agriculture and Livestock. Republic of Turkey Ministry of Food Agriculture and Livestock GAP International Agricultural Research and Training Center for Grain Report. Diyarbakır; 2013. https://arastirma.tarimorman.gov.tr/gaputaem/Belgeler/tarımsalveriler/gaputaemgncel/TahılRaporu.pdf . Accessed 29 Jan 2022.
Download references
Acknowledgments
The authors are grateful for help and support provided by Prof. Dr. Damla Gökşen Şimşek, Assoc. Prof. Dr. Aslı Aslan, Spec. Dr. Eren Er and all health professionals from Ege University who enabled contact with potential cases and controls during the data collection.
Not applicable.
Author information
Authors and affiliations.
Department of Nutrition and Dietetics, Faculty of Health Sciences, Acibadem University, İstanbul, Turkey
İpek Çiçekli
Department of Public Health, Faculty of Medicine, Ege University, İzmir, Turkey
Raika Durusoy
You can also search for this author in PubMed Google Scholar
Contributions
R.D., and İ.Ç. contributed to the design and implementation of the research and to the analysis of the results. İ.Ç. contributed to the writing of the manuscript. R.D. encouraged İ.Ç. to investigate and she supervised this work. All authors read and approved the final manuscript for submission.
Corresponding author
Correspondence to İpek Çiçekli .
Ethics declarations
Ethics approval and consent to participate.
The research was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ege University Faculty of Medicine Clinical Research Ethics Committee (decision no.10059, dated 9 January 2020). Written approval was obtained from the administration of the Department of Child Health and Diseases before starting the study.
Consent for publication
Competing interests.
We have no known conflict of interest to disclose.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Çiçekli, İ., Durusoy, R. Breastfeeding, nutrition and type 1 diabetes: a case-control study in Izmir, Turkey. Int Breastfeed J 17 , 42 (2022). https://doi.org/10.1186/s13006-022-00470-z
Download citation
Received : 09 June 2021
Accepted : 27 March 2022
Published : 27 May 2022
DOI : https://doi.org/10.1186/s13006-022-00470-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Diabetes mellitus
- Risk factors
International Breastfeeding Journal
ISSN: 1746-4358
- General enquiries: [email protected]
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- PubMed/Medline
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Environmental factors associated with type 1 diabetes development: a case control study in egypt.

1. Introduction
2. subjects and methods, 2.1. study tools, 2.2. data entry and analysis, 3.1. description of the study sample, 3.2. maternal and environmental factors occurring during pregnancy, 3.3. natal and neonatal factors, 3.4. feeding practices in the first year of life, 3.5. childhood environmental factors, 4. discussion, 5. conclusions, acknowledgment, author contributions, conflicts of interest.
- Atkinson, M.A.; Eisenbarth, G.S. Type 1 diabetes: New perspectives on disease pathogenesis and treatment. Lancet 2001 , 358 , 221–229. [ Google Scholar ] [ CrossRef ]
- Patterson, C.; Guariguata, L.; Dahlquist, G.; Soltész, G.; Ogle, G.; Silink, M. Diabetes in the young—A global view and worldwide estimates of numbers of children with type 1 diabetes. Diabetes Res. Clin. Pract. 2014 , 103 , 161–175. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ahadi, M.; Tabatabaeiyan, M.; Moazzami, K. Association between environmental factors and risk of type 1 diabetes—A case-control study. Endokrynol. Pol. 2011 , 62 , 134–137. [ Google Scholar ] [ PubMed ]
- Stene, L.C.; Magnus, P.; Lie, R.T.; Søvik, O.; Joner, G.; Group, N.C.D.S. Birth weight and childhood onset type 1 diabetes: Population based cohort study. BMJ 2001 , 322 , 889–892. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Åkerblom, H.K.; Vaarala, O.; Hyöty, H.; Ilonen, J.; Knip, M. Environmental factors in the etiology of type 1 diabetes. Am. J. Med. Genet. 2002 , 115 , 18–29. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Craig, M.E.; Nair, S.; Stein, H.; Rawlinson, W.D. Viruses and type 1 diabetes: A new look at an old story. Pediatr. Diabetes 2013 , 14 , 149–158. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cardwell, C.R.; Stene, L.C.; Joner, G.; Bulsara, M.K.; Cinek, O.; Rosenbauer, J.; Ludvigsson, J.; Jané, M.; Svensson, J.; Goldacre, M.J. Maternal age at birth and childhood type 1 diabetes: A pooled analysis of 30 observational studies. Diabetes 2010 , 59 , 486–494. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cardwell, C.; Stene, L.; Joner, G.; Cinek, O.; Svensson, J.; Goldacre, M.; Parslow, R.; Pozzilli, P.; Brigis, G.; Stoyanov, D.; et al. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: A meta-analysis of observational studies. Diabetologia 2008 , 51 , 726–735. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cardwell, C.R.; Stene, L.C.; Ludvigsson, J.; Rosenbauer, J.; Cinek, O.; Svensson, J.; Perez-Bravo, F.; Memon, A.; Gimeno, S.G.; Wadsworth, E.J.; et al. Breast-feeding and childhood-onset type 1 diabetes. Diabetes Care 2012 , 35 , 2215–2225. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zipitis, C.S.; Akobeng, A.K. Vitamin D supplementation in early childhood and risk of type 1 diabetes: A systematic review and meta-analysis. Arch. Dis. Child. 2008 , 93 , 512–517. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kaila, B.; Taback, S.P. The effect of day care exposure on the risk of developing type 1 diabetes. Diabetes Care 2001 , 24 , 1353–1358. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chadha, V. Sample size determination in health studies. NTI Bull. 2006 , 42 , 55–62. [ Google Scholar ]
- World Health Organization. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus ; World Health Organization: Geneva, Switzerland, 2011. [ Google Scholar ]
- Rytkönen, M.; Moltchanova, E.; Ranta, J.; Taskinen, O.; Tuomilehto, J.; Karvonen, M. The incidence of type 1 diabetes among children in Finland—Rural-urban difference. Health Place 2003 , 9 , 315–325. [ Google Scholar ] [ CrossRef ]
- Liese, A.D.; Puett, R.C.; Lamichhane, A.P.; Nichols, M.D.; Dabelea, D.; Lawson, A.B.; Porter, D.E.; Hibbert, J.D.; D’Agostino, R.B.; Mayer-Davis, E.J. Neighborhood level risk factors for type 1 diabetes in youth: The SEARCH case-control study. Int. J. Health Geogr. 2012 , 11 , 1. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Menon, V.U.; Kumar, K.V.; Gilchrist, A.; Sugathan, T.; Sundaram, K.; Nair, V.; Kumar, H. Prevalence of known and undetected diabetes and associated risk factors in central Kerala—ADEPS. Diabetes Res. Clin. Pract. 2006 , 74 , 289–294. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cherubini, V.; Carle, F.; Gesuita, R.; Iannilli, A.; Tuomilehto, J.; Prisco, F.; Iafusco, D.; Altobelli, E.; Chiarelli, F.; De Giorgi, G. Large incidence variation of type I diabetes in central-southern Italy 1990–1995: Lower risk in rural areas. Diabetologia 1999 , 42 , 789–792. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- El-Hefnawi, A. Protecting agricultural land from urbanization or managing the conflict between informal urban growth while meeting the demands of the communities. In Third Urban Research Symposium on Land Development, Urban Policy and Poverty Reduction, Brasilia, Brazil ; World Bank Institute of Applied Economic Research (IPEA): Brasilia, Brazil, 2005. [ Google Scholar ]
- Marshall, A.; Chetwynd, A.; Morris, J.; Placzek, M.; Smith, C.; Olabi, A.; Thistlethwaite, D. Type 1 diabetes mellitus in childhood: A matched case control study in Lancashire and Cumbria, UK. Diabet. Med. 2004 , 21 , 1035–1040. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sipetic-Grujicic, S.; Vlajinac, H.; Kocev, N.; Marinkovic, J. Family history and risk of type 1 diabetes mellitus. Arch. Dis. Child. 2008 , 93 , 111–115. [ Google Scholar ]
- Svensson, J.; Carstensen, B.; Mortensen, H.B.; Borch-Johnsen, K. Early childhood risk factors associated with type 1 diabetes—Is gender important? Eur. J. Epidemiol. 2005 , 20 , 429–434. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Patelarou, E.; Girvalaki, C.; Brokalaki, H.; Patelarou, A.; Androulaki, Z.; Vardavas, C. Current evidence on the associations of breastfeeding, infant formula, and cow’s milk introduction with type 1 diabetes mellitus: A systematic review. Nutr. Rev. 2012 , 70 , 509–519. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Robertson, L.; Harrild, K. Maternal and neonatal risk factors for childhood type 1 diabetes: A matched case-control study. BMC Public Health 2010 , 10 , 281. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- McNamee, M.B.; Cardwell, C.R.; Patterson, C.C. Neonatal jaundice is associated with a small increase in the risk of childhood type 1 diabetes: A meta-analysis of observational studies. Acta Diabetol. 2012 , 49 , 83–87. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sadeharju, K.; Knip, M.; Virtanen, S.M.; Savilahti, E.; Tauriainen, S.; Koskela, P.; Åkerblom, H.K.; Hyöty, H.; Finnish TRIGR Study Group. Maternal antibodies in breast milk protect the child from enterovirus infections. Pediatrics 2007 , 119 , 941–946. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hyppönen, E.; Kenward, M.G.; Virtanen, S.M.; Piitulainen, A.; Virta-Autio, P.; Tuomilehto, J.; Knip, M.; Akerblom, H.K. Infant feeding, early weight gain, and risk of type 1 diabetes. Childhood Diabetes in Finland (DiMe) Study Group. Diabetes Care 1999 , 22 , 1961–1965. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Virtanen, S.M.; Knip, M. Nutritional risk predictors of β cell autoimmunity and type 1 diabetes at a young age. Am. J. Clin. Nutr. 2003 , 78 , 1053–1067. [ Google Scholar ] [ PubMed ]
- Vaarala, O.; Knip, M.; Paronen, J.; Hämäläinen, A.-M.; Muona, P.; Väätäinen, M.; Ilonen, J.; Simell, O.; Akerblom, H.K. Cow’s milk formula feeding induces primary immunization to insulin in infants at genetic risk for type 1 diabetes. Diabetes 1999 , 48 , 1389–1394. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Vaarala, O. The gut immune system and type 1 diabetes. Ann. N. Y. Acad. Sci. 2002 , 958 , 39–46. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gregori, S.; Giarratana, N.; Smiroldo, S.; Uskokovic, M.; Adorini, L. A 1α, 25-dihydroxyvitamin D3 analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes 2002 , 51 , 1367–1374. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Knip, M.; Veijola, R.; Virtanen, S.M.; Hyöty, H.; Vaarala, O.; Åkerblom, H.K. Environmental triggers and determinants of type 1 diabetes. Diabetes 2005 , 54 , S125–S136. [ Google Scholar ] [ CrossRef ] [ PubMed ]
| Variables | Cases n = 204 | Controls n = 204 |
|---|---|---|
| Sex | ||
| Male | 100 (49.0%) | 100 (49.0%) |
| Female | 104 (51.0%) | 104 (51.0%) |
| Age (years) | ||
| 6–9 | 116 (56.86%) | 116 (56.86%) |
| 10–16 | 88 (43.14%) | 88 (43.14%) |
| Age at diagnosis of T1DM (years) | 7.94 ± 3.39 | - |
| Duration of T1DM (years) | 6.36 ± 4.46 | - |
| Maternal Factors | aOR (95% CI) |
|---|---|
| Residence (rural vs. urban) | |
| Family income (insufficient vs. sufficient) | |
| Mother’s age (>35 years vs. <35 years) | |
| Family member smoking (positive history vs. negative) | 1.01 (0.85–1.89) |
| Family history of type 1 DM (positive history vs. negative) | |
| Consanguinity (positive history vs. negative) | |
| History of maternal diseases/exposures during pregnancy | |
| Pre-eclampsia (positive vs. negative) | 1.83 (0.26–7.83) |
| Gestational DM (positive vs. negative) | 0.98 (0.38–2.52) |
| Maternal infection (positive vs. negative) | |
| Antepartum hemorrhage (positive vs. negative) | 1.21 (0.43–3.40) |
| Over weight gain during pregnancy (positive vs. negative) | |
| Antibiotic intake during pregnancy (positive vs. negative) | 2.45 (0.59–10.98) |
| Antihypertensive intake during pregnancy (positive vs. negative) | 1.50 (0.26–8.62) |
| Antiemetic intake during pregnancy (positive vs. negative) | 0.93 (0.43–2.15) |
| Natal and Neonatal Factors | aOR (95% CI) |
|---|---|
| Place of delivery (hospital vs. home) | |
| Mod of delivery (cesarean vs. vaginal) | |
| Duration of pregnancy (preterm vs. full-term) | |
| Birth weight (Kg) | |
| Birth weight (2.5–3.0 vs. <2.5) | 0.77 (0.30–2.01) |
| Birth weight (>3 vs. <2.5) | 0.94 (0.34–2.62) |
| Birth order | |
| Birth order (second vs. first) | |
| Birth order (third or more vs. first) | |
| History of neonatal jaundice (positive vs. negative) | |
| History of neonatal infection (positive vs. negative) | 0.34 (0.07–1.68) |
| History of neonatal respiratory distress (positive vs. negative) | 0.43 (0.09–1.56) |
| Feeding Practice | aOR (95% CI) |
|---|---|
| Breast feeding (>6 months vs. <6 months) | |
| History of introduction of cow’s milk in first year of life (positive vs. negative) | |
| History of vitamin D Supplementation in first year of life (positive vs. negative) | |
| Onset of weaning (less than five months vs. more than five months) | 1.25 (0.24–1.71) |
| Childhood Environmental Factors | aOR (95% CI) |
|---|---|
| Rubella | 1.02 (0.01–86.72) |
| Measles | 7.89 (0.49–127.14) |
| Varicella | 4.62 (0.59–13.42) |
| Mumps | 3.65 (0.56–20.53) |
| Eczema | 1.88 (0.61–5.74) |
| Rhinitis and conjunctivitis | 1.39 (0.45–4.29) |
| Bronchial asthma | 1.33 (0.38–4.71) |
| Frequent intake of preserved meat (>4 times/week) | 0.59 (0.36–1.95) |
| Frequent intake of sweets (high) | |
| Frequent intake of meat (>4 times/week) | 1.25 (0.52–3.01) |
| Frequent Intake of fish (>4 times/week) | 3.66 (0.77–17.35) |
| Frequent Intake of vegetables (>4 times/week) | |
| Moving home (positive history vs. negative) | 1.75 (0.11–27.10) |
| Physical activity (high vs. low) |
| Factors | β | S.E. | aOR (95% CI) |
|---|---|---|---|
| Residence (rural vs. urban) | 0.79 | 0.36 | |
| Parental history of T1DM (positive history vs. negative) | 3.90 | 2.54 | |
| Mod Of Delivery (cesarean vs. vaginal) | 0.75 | 0.43 | |
| Breast feeding (>6 months vs. <6 months) | −1.46 | 0.39 | |
| Introduction of cows milk in first year of life (positive vs. negative) | 2.99 | 0.42 | |
| Vitamin D Supplementation in first year of life (positive vs. negative) | −2.16 | 0.37 | |
| Physical Activity (high vs. low) | −2.90 | 0.44 |
Share and Cite
Awadalla, N.J.; Hegazy, A.A.; Abd El-Salam, M.; Elhady, M. Environmental Factors Associated with Type 1 Diabetes Development: A Case Control Study in Egypt. Int. J. Environ. Res. Public Health 2017 , 14 , 615. https://doi.org/10.3390/ijerph14060615
Awadalla NJ, Hegazy AA, Abd El-Salam M, Elhady M. Environmental Factors Associated with Type 1 Diabetes Development: A Case Control Study in Egypt. International Journal of Environmental Research and Public Health . 2017; 14(6):615. https://doi.org/10.3390/ijerph14060615
Awadalla, Nabil J., Amal A. Hegazy, Manal Abd El-Salam, and Marwa Elhady. 2017. "Environmental Factors Associated with Type 1 Diabetes Development: A Case Control Study in Egypt" International Journal of Environmental Research and Public Health 14, no. 6: 615. https://doi.org/10.3390/ijerph14060615
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
Advertisement
A case–control study to evaluate irisin levels in patients with type 1 diabetes mellitus
- Original Article
- Published: 13 February 2024
- Volume 193 , pages 1275–1282, ( 2024 )
Cite this article

- Dhifaf A. Abdulabbas 1 &
- Ekhlas Abdallah Hassan ORCID: orcid.org/0000-0001-7655-6230 1
170 Accesses
Explore all metrics
Irisin is a precious hormone-like myokine that plays a key role in glucose/energy expenditure and metabolic regulation This paper aimed to determine the irisin levels in patients with type 1 diabetes mellitus and their correlation with insulin therapy and glycaemic control.
Ninety type 1 diabetes mellitus patients were collected. The patients were subdivided into two groups: group I (37) newly diagnosed type 1 diabetes mellitus and group II (53) T1DM (on insulin injection); for comparison, 30 healthy individuals were included as control. The serum levels of irisin were estimated using ELISA. FSG and lipid profile were measured through spectrophotometrically. Glycated hemoglobin was determined using High-performance liquid chromatography.
Serum levels of irisin were significantly lower ( P = 0.01), as compared to the control group. Also irisin level was significantly lower in group I compared to group II. Fasting serum glucose, glycated hemoglobin, and lipid profile were significantly elevated in patient groups compared to the control group. Serum irisin was negatively correlated to fasting serum glucose, and glycated hemoglobin, whereas it positively correlated to serum lipid profile. In multiple stepwise regression, only glycated hemoglobin ( β = − 0.600, P = 0.040) was determined as an independent predictor for predicting the irisin levels. The AUC was excellent (AUC = 0.996, P = 0.0001), with high diagnostic accuracy (88.2) in differentiating newly diagnosed type 1 diabetes mellitus from the healthy subject group.
We demonstrated low irisin levels in type 1 diabetes mellitus and the association of the highest irisin amounts to an insulin therapy and a better glycaemic control. Furthermore, the measurement of irisin levels could be useful as laboratory markers to monitor type 1 diabetes mellitus severity and therapy response.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or Ebook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD)

Introduction to Diabetes Mellitus

Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD)
Data availability.
(ROC) analysis revealed the diagnostic accuracy of using irisin concentrations to distinguish patient groups from healthy control subjects.
Abbreviations
- Type 1 diabetes mellitus
Fasting blood sugar
Glycated hemoglobin
Body mass index
High-density lipoprotein
Low density lipoprotein
Receiver operating characteristic curve
Total cholesterol
Triglyceride
Very low-density lipoprotein
Jaid HK, Khaleel FM, Salman IN, Abd BA (2023) Estimation of apelin levels in Iraqi patients with Type II diabetic peripheral neuropathy. Baghdad Sci J 20(5):1684–1691
Google Scholar
Abed BA, Farhan LO, Dawood AS (2024) Relationship between serum nesfatin-1, adiponectin, resistin concentration, and obesity with type 2 diabetes mellitus. Baghdad Sci J 21(1):0117
Cho NH, Shaw JE, Karuranga S et al (2018) IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138:271–281
Article CAS PubMed Google Scholar
Shaban A, Abbas SA, Abed BA (2023) Estimation of Tenascin-C Levels in Iraqi patients with diabetic nephropathy. Al-Rafidain J Med Sci 5(1S):S8–13. ISSN 2789-3219
Article Google Scholar
Guariguata L (2011) Estimating the worldwide burden of type 1 diabetes. Diabetes Voice 56(2):6–8
Rana KS, Pararasa C, Afzal I et al (2017) Plasma irisin is elevated in type 2 diabetes and is associated with increased E-selectin levels. Cardiovasc Diabetol 16:147. https://doi.org/10.1186/s12933-017-0627-2
Article CAS PubMed PubMed Central Google Scholar
Arhire LI, Mihalache L, Covasa M (2019) Irisin: a hope in understanding and managing obesity and metabolic syndrome. Front Endocrinol (Lausanne) 10:524
Article PubMed Google Scholar
Perakakis N, Triantafyllou GA, Fernández-Real JM et al (2017) Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol 13(6):324–337. https://doi.org/10.1038/nrendo.2016.221
Alis R, Sanchis Gomar F, Pareja Galeano H et al (2014) Association between irisin and homocysteine in euglycemic and diabetic subjects. Clin Biochem 47(18):333–35
Choi YK, Kim MK, Bae KH et al (2013) Serum irisin levels in new-onset type 2 diabetes. Diabetes Res Clin ract 100(1):96–101
Article CAS Google Scholar
Diabetes A (2012) Association Diagnosis and classification of diabetes mellitus. Diabet Care 36:S67–S74
Fossati P, Prencipe L (1982) Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem 28(10):2077–2080
Richmond W (1974) Proceedings in the development of an enzymatic technique for the assay of cholesterol in biological fluids. Clin Sci Mol Med 46(1):6P–7P
Naito HK (1984) High-density lipoprotein (HDL) cholesterol. In: Kaplan A et al. Clin Chem. C.V. Mosby Co., St Louis, pp 1207–1213 and 437
Boström P1, Wu J, Jedrychowski MP et al (2012) A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481:463–468
Article PubMed PubMed Central Google Scholar
Chen N, Li Q, Liu J, Jia S (2016) Irisin, an exercise-induced myokine as a metabolic regulator: an updated narrative review. Diabetes Metab Res Rev 32:51–59
Natalicchio A, Marrano N, Biondi G et al (2017) The myokine irisin is released in response to saturated fatty acids and promotes pancreatic β-cell survival and insulin secretion. Diabetes 66:2849–2856
Xiong XQ, Chen D, Sun HJ et al (2015) FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim Biophys Acta 1852:1867–1875
Faienza MF, Brunetti G, Sanesi L et al (2018) High irisin levels are associated with better glycemic control and bone health in children with Type 1 diabetes. Diabetes Res Clin Pract 1(141):10–17
Ates I, Arikan MF, Erdogan K et al (2017) Factors associated with increased irisin levels in the type 1 diabetes mellitus. Endocr Regul 51(1):1–7
Yang Y, Xie M, Yuan S et al (2021) Sex differences in the associations between adiposity distribution and cardiometabolic risk factors in overweight or obese individuals: a cross-sectional study. BMC Public Health 21:1232. https://doi.org/10.1186/s12889-021-11316-4
Yang Y, Xie M, Yuan S et al (2021) Sex differences in the associations between adiposity distribution and cardiometabolic risk factors in overweight or obese individuals: a cross-sectional study. BMC Public Health 21(1):1232
Espes D, Lau J, Carlsson PO (2015) Increased levels of irisin in people with long-standing type 1 diabetes. Diabet Med 32:1172–1176
Download references
Acknowledgements
We owe our deepest gratitude to the University of Diyala, College of Science.
Author information
Authors and affiliations.
Chemistry Department, College of Science, Diyala University, Baquba, Diyala, Iraq
Dhifaf A. Abdulabbas & Ekhlas Abdallah Hassan
You can also search for this author in PubMed Google Scholar
Contributions
Dhifaf A. Abdulabbas: methodology, software, and data curation. Ekhlas Abdallah Hassan: conceptualization, writing—original draft preparation, visualization, investigation , software, validation, writing—reviewing and editing.
Corresponding author
Correspondence to Ekhlas Abdallah Hassan .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
• Irisin is a new biomarker candidate in type 1 diabetes mellitus.
• Irisin can be used to differentiate newly diagnosed T1DM from healthy subjects.
• Irisin change depends on the severity of diseases (disease severity depends on increased HbA1c) of T1DM.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Abdulabbas, D.A., Hassan, E.A. A case–control study to evaluate irisin levels in patients with type 1 diabetes mellitus. Ir J Med Sci 193 , 1275–1282 (2024). https://doi.org/10.1007/s11845-024-03626-4
Download citation
Received : 12 January 2024
Accepted : 01 February 2024
Published : 13 February 2024
Issue Date : June 2024
DOI : https://doi.org/10.1007/s11845-024-03626-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Glycaemic control
- Insulin therapy
- Newly diagnosed
- Find a journal
- Publish with us
- Track your research
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- BMJ Journals
You are here
- Volume 12, Issue 1
- Use of machine learning to identify characteristics associated with severe hypoglycemia in older adults with type 1 diabetes: a post-hoc analysis of a case–control study
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0002-9905-4855 Nikki L B Freeman 1 ,
- Rashmi Muthukkumar 2 ,
- Ruth S Weinstock 3 ,
- M Victor Wickerhauser 4 ,
- http://orcid.org/0000-0003-2701-101X Anna R Kahkoska 5 , 6
- 1 Department of Surgery , University of North Carolina at Chapel Hill School of Medicine , Chapel Hill , North Carolina , USA
- 2 Department of Medicine , University of North Carolina at Chapel Hill , Chapel Hill , North Carolina , USA
- 3 Department of Medicine , SUNY Upstate Medical University , Syracuse , New York , USA
- 4 Department of Mathematics , Washington University in St Louis , St Louis , Missouri , USA
- 5 Department of Nutrition , University of North Carolina at Chapel Hill , Chapel Hill , North Carolina , USA
- 6 Division of Endocrinology and Metabolism , University of North Carolina at Chapel Hill , Chapel Hill , North Carolina , USA
- Correspondence to Dr Nikki L B Freeman; nlbf{at}live.unc.edu
Introduction Severe hypoglycemia (SH) in older adults (OAs) with type 1 diabetes is associated with profound morbidity and mortality, yet its etiology can be complex and multifactorial. Enhanced tools to identify OAs who are at high risk for SH are needed. This study used machine learning to identify characteristics that distinguish those with and without recent SH, selecting from a range of demographic and clinical, behavioral and lifestyle, and neurocognitive characteristics, along with continuous glucose monitoring (CGM) measures.
Research design and methods Data from a case–control study involving OAs recruited from the T1D Exchange Clinical Network were analyzed. The random forest machine learning algorithm was used to elucidate the characteristics associated with case versus control status and their relative importance. Models with successively rich characteristic sets were examined to systematically incorporate each domain of possible risk characteristics.
Results Data from 191 OAs with type 1 diabetes (47.1% female, 92.1% non-Hispanic white) were analyzed. Across models, hypoglycemia unawareness was the top characteristic associated with SH history. For the model with the richest input data, the most important characteristics, in descending order, were hypoglycemia unawareness, hypoglycemia fear, coefficient of variation from CGM, % time blood glucose below 70 mg/dL, and trail making test B score.
Conclusions Machine learning may augment risk stratification for OAs by identifying key characteristics associated with SH. Prospective studies are needed to identify the predictive performance of these risk characteristics.
- Severe Hypoglycemia
- Diabetes Mellitus, Type 1
- Case-Control Studies
Data availability statement
The de-identified study datasets are available in a public, open-access repository.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjdrc-2023-003748
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Severe hypoglycemia in older adults with type 1 diabetes is associated with significant morbidity and mortality, and previous work by Weinstock et al identified the characteristics that distinguish older adults with a recent history of severe hypoglycemia from those without across a wide range of potentially important variables.
WHAT THIS STUDY ADDS
This study aimed to harness machine learning methods to uncover the relative importance of those variables, including demographic, clinical, lifestyle, and neurocognitive characteristics, and continuous glucose monitoring (CGM) measures associated with a history of severe hypoglycemia among older adults with type 1 diabetes.
The individual-level characteristics associated with a history of severe hypoglycemia were hypoglycemia unawareness, hypoglycemia fear, glycemic variability as measured by CGM (coefficient of variation), % time blood glucose below 70 mg/dL, and trail making test B score.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study shows how machine learning models can be applied to prioritize risk characteristics for severe hypoglycemia.
The results may inform future risk stratification tools for older adults designed to aid care providers in providing data-driven, individualized counseling related to severe hypoglycemia.
Introduction
Older adults with type 1 diabetes are a growing population within the USA. 1 Although hypoglycemia is a concern at any age for people with type 1 diabetes, older adults are at substantially higher risk of hypoglycemia compared with younger adults. It has previously been reported that the incidence of one or more episodes of severe hypoglycemia (SH) in patients over age 65 within a 12-month period is 16.1%. 2 For older adults who have had type 1 diabetes for 40 years or more, the incidence of SH can be as high as 18.6% within 1 year. 2 Another study has shown that older adults over age 60 with type 1 diabetes have double the risk for SH compared with their younger counterparts. 3
Among older adults, episodes of SH are associated with significant morbidity, including hospitalization, falls, fractures, altered mentation, and seizures. 1 4 In addition to the acute effects of hypoglycemia, in older adults the risk of other long-term side effects is also increased, including decreases in cognitive function. 5 SH episodes may affect cognition in older adults with type 1 diabetes related to language, executive function, and episodic memory, potentially because the brains of older adults are more susceptible to harm from SH compared with younger adults. 6 Avoiding these episodes is thus a priority of care. 7
The increasing risk of SH with age may be attributed to changes to cognitive status, 8 metabolism and insulin sensitivity, higher prevalence rates of hypoglycemia unawareness, frailty, and functional impairments, as well as polypharmacy. 7 However, a challenge for mitigating the risk of SH on an individual level is that its etiology among older adults is both complex and multifactorial. Previous work by Weinstock et al 4 collected comprehensive data from older adults with type 1 diabetes in a case–control design and found that SH events were associated with increased hypoglycemia unawareness and glucose variability, with cases and controls having similar mean hemoglobin A1c (HbA1c) and mean continuous glucose monitoring (CGM)-measured glucose levels. Given that there are potentially many characteristics which may impact the risk for SH, spanning demographic and clinical characteristics, behavioral and lifestyle characteristics, neurocognitive characteristics, and CGM measures, there is a need to identify singular and sets of individual-level characteristics which may serve to identify older adults at high risk of SH.
Machine learning methods can “mine” high-dimensional data to uncover complex relationships between multiple potential risk characteristics and outcomes with fewer assumptions than traditional methods. We hypothesized that these methods could complement findings from traditional statistical analyses such as those by Weinstock et al 4 to provide insight into how different risk characteristics may be prioritized in a clinical setting to identify older adults at highest risk for SH; this is an important step toward risk stratification to tailor efforts to reduce significant morbidity and possible mortality associated with SH in this population. Building on the work of Weinstock et al that identified factors to distinguish older adults with a recent history of SH from those without from a wide range of potentially important variables, this study aimed to understand the relative importance of those characteristics. The long-term objective of this study is to generate new insights that may improve clinical tools for enhanced risk stratification to identify older adults at risk of SH.
Research design and methods
Study design.
This study used a data set from a prior case–control study to identify risk characteristics for SH in older adults with type 1 diabetes. 4 The random forest algorithm was used to classify (ie, identify) cases versus controls based on individual-level characteristics (ie, covariates). Participants in the original case–control study consented to taking part in the study. 4
Data source
The data set for this study was initially used by Weinstock et al to identify risk characteristics for SH in older adults age 60 and older with diabetes duration of at least 20 years. 4 9 The original study was a case–control study with 201 participants from 18 T1D Exchange Clinical Network centers. 10 Cases were participants who reported an SH event within the past 12 months of study participation and controls did not have SH in the past 3 years. An SH event was defined as a hypoglycemic event leading to altered mentation or loss of consciousness and requiring the assistance of another individual to provide resuscitative assistance through carbohydrates, glucagon, or other means. Potential participants were excluded if they were current CGM users, recipients of pancreatic transplants, with life expectancy of less than 1 year, moderate or advanced dementia, or chronic kidney disease with a glomerular filtration rate of less than 30 mL/min/1.73 m 2 . 4
All data collection procedures are described in detail in Weinstock et al . 4 Demographic variables of interest included sex, race/ethnicity, education level, insurance type, and household size. Race/ethnicity was included as a social construct rather than to reflect differences in biology. Clinical variables of interest included body mass index, exercise, frequency of blood glucose monitoring, mean daytime and nocturnal blood glucose from CGM and blood glucose variability, HbA1c, insulin delivery system and dosing, medications, C peptide levels, creatinine, and hospitalization for diabetic ketoacidosis. Information on cognition, psychomotor skills, frailty, fear of hypoglycemia, hypoglycemia unawareness, and social support was also collected using a variety of survey and physical testing methods, described in the following.
Hypoglycemia unawareness was measured using the Clarke Hypoglycemia Awareness Questionnaire. 11 As noted in Weinstock et al , 4 the Clarke questionnaire includes questions about recent hypoglycemic events, which invalidates the use of the total score for this analysis. More recently, the Clarke score has been deconstructed into two subscales: SH experience and hypoglycemia awareness status. 12 To proxy hypoglycemia unawareness, removed from history of SH, we generated a raw score for the questionnaire elements that measures hypoglycemia awareness status and excluded the items that measure SH experience. Fear of hypoglycemia was assessed using the Hypoglycemia Fear Survey. 13 Neurocognitive testing was completed twice, 2 weeks apart. Mental status testing included the Montreal Cognitive Assessment. 14 Psychomotor testing was completed using the Symbol Digit Modalities Test. 15 Executive functioning was done using two trail making tests (trail making tests A and B). 16 17 Verbal memory was tested using the Hopkins Verbal Learning Test. 18 The grooved pegboard test was used to assess fine motor dexterity and speed. 19 Social support was assessed using the Duke Social Support Index. 20 Frailty was assessed using the timed 10-foot walk test. CGM data were blinded in the original study with SEVEN PLUS CGM devices worn by participants for 14 days with calibration daily. CGM was worn on average for 277 hours by case participants and 294 hours by control participants. The specific way in which each of these variables was operationalized in the model is shown in online supplemental table S1 .
Supplemental material
Statistical analysis.
Successively complex (ie, richer) models were examined to incorporate demographic and clinical characteristics, behavioral and lifestyle characteristics, neurocognitive characteristics, and CGM measures ( figure 1 ). The rationale for this approach was to use clinically accessible measures for model 1, exclusively. Subsequent models, models 2–4, include measures that require more time, tools, or resources to collect.
- Download figure
- Open in new tab
- Download powerpoint
Models. We tested four models that were successively more complex, incorporating more individual-level characteristics that may be associated with severe hypoglycemia. BMI, body mass index; CGM, continuous glucose monitoring; CV, coefficient of variation; HbA1c, hemoglobin A1c.
Stratified by case–control status and for the overall study population, we used descriptive statistics to summarize the characteristics of the study population. Binary and categorical characteristics were described using counts and percentages; numerical characteristics were described using the median, minimum, and maximum.
Because of missingness in the variables, we implemented multiple imputation 21 on the full analytic data set. Multiple imputation models the missing values conditional on the observed values. The model, in turn, is used to impute multiple likely values for the missing values and thus yields multiple imputed data sets. For our analysis, we generated 10 imputation data sets.
After multiple imputation, we split the observations into those to be included in the testing data set (test set) and those to be included in the training data set (training set). Observations included in the test set were chosen by randomly selecting 40% of the observations with complete data. Observations not in the test set were included in the training set. Overall, the train–test split was 78%/22%. Because observations included in the test set were complete cases, that is, information across the imputation data sets for the test cases was identical, the test set was exactly the single data set consisting of data for the test cases. In contrast, the training set consisted of the ten multiple imputation data sets subsetted on the observations selected as training cases.
To mitigate overfitting, we used feature selection techniques before fitting the machine learning models. First, we used correlation matrices to identify redundant characteristics and considered characteristics with an absolute correlation greater than 0.75 as redundant. Second, we used recursive feature elimination to identify the characteristics to include in our models to optimize accuracy.
Random forests 22 were trained on the training set and assessed on classification performance. For each of the four models considered, we fit a random forest on each of the imputation data sets in the training set. A random forest is a machine learning method that can be characterized as an ensemble of weak learners. 23 In the case of random forests, the weak learners are simple trees—the trees are the “learners,” or models, and they are called weak because individual trees on average have poor predictive power and performance. Ensembling, in the context of random forests, means that many trees are constructed and each tree “votes” to contribute to the final prediction yielded from the forest of trees. The “random” part of random forests refers to the injection of randomness in tree construction, for example, which characteristics are included in the tree and which ensures that the forest of trees has some heterogeneity and in turn improves performance. In our analysis, for each model, after training (ie, fitting) a random forest to each imputation set, we use the fitted model to classify observations in the test set as cases or controls. To assess model performance, sensitivity, specificity, and precision were calculated and averaged across the imputation sets.
Random forests naturally generate variable importance. In our analysis, we used the mean decrease in the Gini index to identify the importance of each variable. This metric is based on the idea of node purity. A node in a decision tree is a split point and each split is based on a variable. Node purity is a measure of the homogeneity of the labels at a particular node; the more homogeneous the labels the purer the node. The mean decrease in the Gini index captures the extent to which a particular variable, on average, decreases the impurity of a split among the constituent trees, or equivalently, the information gain from the use of that particular variable. The larger the mean decrease in the Gini index, the more important the variable across trees in the random forest.
Data and resource availability
The data set analyzed in the current study is publicly available from the Jaeb Center for Health Research database at https://public.jaeb.org/datasets/diabetes . 9 Analyses were conducted using the R statistical programming language. 24 The mice package 25 was used for multiple imputation, the caret package 26 was used to construct the test and training sets, and the randomForest package 27 was used to train the random forests. Git was used for version control; the code repository is stored on GitHub ( https://github.com/nikkifreeman/T1D_SH_key_predictors ).
Participant characteristics
This study used data for 191 participants from the Weinstock et al data set. Eight cases and four control participants were excluded due to missing demographics (two cases), having less than 7 days of CGM data (three cases), having less than 24 hours of night-time CGM (three cases, three controls), and not having CGM data (one control). The final analytic data set included 95 case participants and 96 controls; their characteristics are described in table 1 .
- View inline
Study participants, by case and control status
The groups were similar in demographic characteristics based on sex, race/ethnicity, education, insurance status, annual income, and household size, with the majority being non-Hispanic white participants between 60 and 75 years old with at least some college education. There were differences between groups related to a variety of clinical characteristics. On average, the case participants monitored their blood glucose more frequently than the control group. Those in the case group had a greater percentage of time with hypoglycemic range blood glucose and had greater variability in their blood glucose measurements throughout the day and night. The case group also scored higher on frailty testing. Conversely, those in the control group scored lower on measures of hypoglycemia unawareness compared with controls. The testing for various functional modalities also showed differences among groups, with those in the control group demonstrating higher cognition, psychomotor skills, and dexterity.
Feature selection and model evaluation
Feature selection procedures revealed redundancy between two variables, the symbol digit modalities written test and the symbol digit modalities oral test. The controlled univariate analysis of the two tests in Weinstock et al 4 indicated a stronger signal for the written test than the oral test (p=0.001 vs p=0.01), so we dropped the oral test score as a covariate in our analysis. Recursive feature elimination did not provide compelling evidence for dropping additional variables from any of our models; thus, no additional variables were eliminated from our analyses (full results in online supplemental figure S2 ). Performance metrics for the fitted random forests, across all four models, are shown in table 2 . The richer models, that is, models 2, 3, and 4, which had more variables as inputs, were more sensitive than model 1, and model 1 was more specific than the richer models. Precision was similar across models 1, 2, 3, and 4.
Random forest model classification performance*
Modeling results
Figure 2 depicts the top five individual-level characteristics associated with having experienced an episode of SH from models 1–4 based on the mean decrease in the Gini index (full results in online supplemental figure S3 ). In model 1, which examined demographic and clinical characteristics, hypoglycemia awareness, HbA1c, glucose monitoring frequency, frailty, and insurance emerged as the most important for discerning between older adults with and without a history of SH. In model 2, where behavioral and lifestyle characteristics were added, hypoglycemia fear and the Duke Social Support Index additionally emerged as key characteristics, displacing frailty and insurance. In model 3, in which neurocognitive characteristics were added, the top five characteristics were hypoglycemia unawareness, hypoglycemia fear, the results of the Symbol Digit Modalities Test (written), the results of the trail making test - test A, and the results of trail making - test B. Finally, in model 4, which additionally included CGM measures, glucose variability as measured by % coefficient of variation and the per cent of time blood glucose below 70 mg/dL emerged as key variables associated with SH history.
Top five characteristics from each model. These are the individual-level characteristics that emerged as most important for discerning between older adults with and without a history of severe hypoglycemia. CGM, continuous glucose monitoring; CV, coefficient of variation; HbA1c, hemoglobin A1c.
We used a machine learning method and data from 191 older adults with type 1 diabetes to identify the individual-level characteristics that were most strongly associated with having experienced an episode of SH, exploring a series of successively complex models using rich and diverse data. We found that when taking into account all possible demographic, clinical, neurocognitive characteristics, and CGM measures, the characteristics associated with a history of SH compared with those who have not had SH were hypoglycemia unawareness, hypoglycemia fear, glycemic variability as measured by CGM (coefficient of variation), the percent of time with blood glucose below 70 mg/dL, and trail making test B score. These results add to the limited literature for older adults with type 1 diabetes and provide a glimpse into the interactions and relative importance of the range of characteristics that are known to contribute to the risk of SH in this age group. Our results point to the important role of hypoglycemia unawareness in the cycle of SH, as well as how shorter-term measures of glycemia and glucose dynamics can be prioritized as part of the set of characteristics associated with long-term risk for SH. Our analysis also underscores the potential utility of incorporating more comprehensive information, including behavioral, neurocognitive, and CGM data, to discern older adult individuals who are at risk for hypoglycemia.
It has been shown that older adults with type 1 diabetes have double the risk of SH compared with their younger counterparts. 3 This is especially true for older adults who have had diabetes for many decades, as the incidence of SH in 1 year increases with longer diabetes duration. 2 As a result, understanding what characteristics put this particularly vulnerable population at increased risk in may help to guide interventions to prevent the potentially devastating impact that SH can have on the health and quality of life of this population. Yet it remains unclear how to make use of diverse input information as part of stratifying older adults with type 1 diabetes based on their risk for SH. 1
To address this gap, our study used the rich demographic and clinical risk characteristics investigated in the study by Weinstock et al 4 to explore potentially complex relationships between those risk characteristics and SH through machine learning modeling. This type of modeling allows for not only the identification of risk characteristics in a more flexible manner than traditional regression style approaches but also to identify the relative importance of those characteristics for SH compared with each other, effectively allowing for prioritization of risk characteristics. The rich data set allowed for the inclusion of characteristics beyond demographic and clinical data to explore behavioral, lifestyle, and neurocognitive risk characteristics associated with SH. Examination of the relative importance of variables in each of the successively rich models illustrates that SH risk is the interplay of characteristics across a multiplicity of domains. Rather than observing characteristics from a single domain, such as clinical characteristics, dominating in importance across models, figure 2 shows that consistently across models the most important characteristics came from a mix of domains. Model sensitivity increased as more characteristic types were included, and the best-performing model in terms of sensitivity was model 4, which incorporated demographic, clinical, behavioral and lifestyle, neurocognitive, and lifestyle characteristics, along with CGM measures, thereby providing a more holistic and detailed view of which characteristics can contribute to SH. Model 4 is important to consider since the complexity of older adults is incompletely captured by their demographic information and basic clinical and laboratory information.
As expected and consistent with Weinstock et al , hypoglycemia unawareness was an important risk characteristic for SH in the random forest modeling. 4 Based on recent studies of the Clark questionnaire, 11 12 we intentionally calculated a score to reflect the construct of hypoglycemia unawareness rather than history of SH. Interestingly, this characteristic remained the most important characteristic associated with SH across all four models and was thus robust to the addition of other information. Physiological changes related to aging such as hormonal response to hypoglycemia can make older adults particularly vulnerable to hypoglycemia unawareness. 28 Weinstock et al 4 also found that fear of hypoglycemia was increased in those with recent SH; this characteristic emerged as a significant characteristic that remained robust across models 2–4, although the temporality of the relationship between this variable and the outcome of SH remains unclear in the case–control design. It is probably that older adults who recently experienced SH reported higher fear as a result of their event.
The vast majority of evidence detailing cognitive function in older adults with diabetes primarily involves those with type 2 diabetes. 8 29 One aspect of the original data set that is particularly interesting was the use of multiple neurocognitive assessments given the significant impact that SH can have on cognition in this population. 5 6 Weinstock et al 4 used the Montreal Cognitive Assessment, the Symbol Digit Modalities Test, the trail making test, and the grooved pegboard test to assess cognition and functioning. There were significant differences among case and control participants related to certain cognitive and functional tests, but it was not possible to elucidate in that study which tests are most predictive in differentiating those at higher risk for SH. The trail making test for executive functioning was a significant characteristic in the original study, and our modeling similarly indicated that trail B was a more significant characteristic compared with the trail A test for executive functioning for case participants. This test for executive functioning has been used in other studies of older adults with type 1 diabetes and those with recent SH did perform worse on that test. 6 Our results advance an understanding of the relative importance of this measure of executive functioning in the context of other potential risk characteristics, underscoring that these characteristics are likely informative in this age group.
Machine learning methods have been used in other studies for a variety of applications for people with type 1 diabetes including for predicting hypoglycemia. Those studies often used CGM data to predict the risk of hypoglycemia in the shorter term. 30–32 Additionally, these usually involved individuals who were younger with shorter diabetes duration and aimed to understand the risk of hypoglycemia in the immediate future based on CGM data. Since the risk of SH increases with increased diabetes duration, it is important to apply these methods to this group as well. While a number of machine learning methods were available for this analysis, we preferred the random forest algorithm because of its ability to naturally select important variables over a method like support vector machines and its ability to handle categorical features better than a method like L1-regularized logistic regression.
Given that the data from this study came from a case–control study, where the case status was based on a retrospective hypoglycemic event, the results provide insight into the characteristics that are robustly associated with SH, rather than true “risk factors” that are associated with acute events in the future. Prospective studies are needed to empirically test the predictive performance of these characteristics, including combinations thereof. A further limitation of this analysis is that participants who regularly use CGM were excluded, which limits generalizability to contemporary populations as CGM or closed-loop systems are becoming more common in older adults. Moreover, those who use CGM may have a different relationship with SH and other risk characteristics that cannot be accurately predicted using this model. In addition, the study used data from 191 participants from the T1D Exchange Clinical Network, a relatively small cohort consisting of a majority of non-Hispanic white participants. 4 As a result, models based on a more diverse population may have different risk characteristics that have contributed more to past SH or show differences in future SH events as in a prospective study. Because of the modest sample size, the number of CGM metrics included in the analysis were limited to those known to be associated with SH risk. Moreover, the variables of age, diabetes duration, or diabetes-related complications were not available in the data set. Including these variables may change the top five key characteristics across all models. Assessments of the social determinants of health were also not included despite the known importance of these variables in diabetes outcomes. 33
The strengths of the study include the use of novel machine learning methods and the ability to compare our findings with prior work to assess for clinical validity of the machine learning models and elucidate how these methods complement traditional regression approaches. There are many possible risk characteristics for SH, and traditional statistical methods, which can help identify whether a characteristic is a risk characteristic or not, may be complemented by machine learning methods that can, for example, provide perspective on the relative importance of those characteristics. The models in this analysis allow for prioritization of potential risk characteristics so clinicians can more efficiently use their appointments to provide more personalized, yet data-driven advice for patients who have similar characteristics to those who have experienced SH. Future work in this space can be used to create risk stratification tools for clinician use. The data set that was used was also an important strength of this analysis because we were able to go beyond simple demographic or clinical measures and explore neurocognitive functioning in addition to CGM measures, thus providing a more holistic picture of the participants involved. Together, these results provide a glimpse into how varying levels of individual-level data can be prioritized in clinical settings to inform discussions with their older adult patients.
Ethics statements
Patient consent for publication.
Not required.
Ethics approval
This study involves human participants. This study analyzed publicly available, deidentified data from a research study that was previously completed. Participants gave informed consent to participate before taking part in the original study. The University of North Carolina at Chapel Hill Institutional Review Board reviewed and approved the study (IRB 23-3227). It was deemed exempt due to being a category 4 study (secondary data/specimens).
- Munshi MN ,
- Meneilly GS ,
- Rodríguez-Mañas L , et al
- Weinstock RS ,
- Maahs DM , et al
- Seufert J , et al
- DuBose SN ,
- Bergenstal RM , et al
- Shigemoto S ,
- Fujisawa R , et al
- Gilsanz P ,
- Eng C , et al
- ElSayed NA ,
- Aroda VR , et al
- JAEB Center for Health Research
- Tamborlane WV ,
- Clarke WL ,
- Gonder-Frederick LA , et al
- Gonder-Frederick LA ,
- Schmidt KM ,
- Vajda KA , et al
- Sepúlveda E ,
- Poínhos R ,
- Nata G , et al
- Tombaugh TN
- Benedict RHB
- ↵ Grooved pegboard test user instructions . Lafayette Instruments ; 2002 .
- Landerman R ,
- George LK ,
- Campbell RT , et al
- Tibshirani R ,
- ↵ R: A language and environment for statistical computing [ R: The R Project for Statistical Computing ]. 2023 . Available : https://www.r-project.org/
- Groothuis-Oudshoorn K
- Martín-Timón I ,
- Del Cañizo-Gómez FJ
- Klein BEK ,
- Lee KE , et al
- Berikov VB ,
- Kutnenko OA ,
- Semenova JF , et al
- DeSalvo DJ ,
- Haridas B , et al
- Tsichlaki S ,
- Koumakis L ,
- Tsiknakis M
- Hill-Briggs F ,
- Berkowitz SA , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
Contributors NLBF and ARK conceived the study. NLBF, MVW, and ARK designed the analysis plan. NLBF conducted the statistical analyses. All authors contributed to the interpretation of the results. RM, NLBF, and ARK prepared the manuscript with contributions from MVW and RW. NLBF is the guarantor of this work.
Funding ARK is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR002490. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. ARK also reports receiving research grants from the Diabetes Research Connection and the American Diabetes Association, and a prize from the National Academy of Medicine, outside the submitted work.
Competing interests RW participated in multicenter clinical trials through her institution, sponsored by Insulet, Medtronic, Eli Lilly, Novo Nordisk, and Boehringer Ingelheim, and has used donated DexCom CGMs and Tandem insulin pumps in projects sponsored by the NIH and the Leona M and Harry B Helmsley Charitable Trust.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
Last updated 09/07/24: Online ordering is currently unavailable due to technical issues. We apologise for any delays responding to customers while we resolve this. For further updates please visit our website: https://www.cambridge.org/news-and-insights/technical-incident
We use cookies to distinguish you from other users and to provide you with a better experience on our websites. Close this message to accept cookies or find out how to manage your cookie settings .
Login Alert

- > Journals
- > Cardiology in the Young
- > Volume 27 Issue 9
- > Type 1 diabetes mellitus and associated risk factors...

Article contents
Type 1 diabetes mellitus and associated risk factors in patients with or without chd: a case–control study.
Published online by Cambridge University Press: 29 May 2017
Approximately 1% of children are born with CHD, and 90–95% reach adulthood. Increased exposure to infections and stress-strain can contribute to an increased risk of developing type 1 diabetes mellitus. CHD may increase the risk of more serious infections, stress-strain, and increased risk of developing type 1 diabetes mellitus.
We analysed the onset of and the risk of mortality and morbidity associated with concurrent CHD in patients with type 1 diabetes mellitus compared with patients with type 1 diabetes mellitus without CHD. The study combined data from the National Diabetes Register and the National Patient Register.
A total of 104 patients with CHD and type 1 diabetes mellitus were matched with 520 controls. Patients with CHD and type 1 diabetes mellitus had an earlier onset of diabetes (13.9 versus 17.4 years, p<0.001), longer duration of diabetes (22.4 versus 18.1 years, p<0.001), higher prevalence of retinopathy (64.0 versus 43.0%, p=0.003), higher creatinine levels (83.5 versus 74.1 µmol/L, p=0.03), higher mortality (16 versus 5%, p=0.002), and after onset of type 1 diabetes mellitus higher rates of co-morbidity (5.28 versus 3.18, p⩽0.01), heart failure (9 versus 2%, p=0.02), and stroke (6 versus 2%, p=0.048) compared with controls.
From a nationwide register of patients with type 1 diabetes mellitus, the coexistence of CHD and type 1 diabetes mellitus was associated with an earlier onset, a higher frequency of microvascular complications, co-morbidity, and mortality.
Access options

This article has been cited by the following publications. This list is generated based on data provided by Crossref .
- Google Scholar
View all Google Scholar citations for this article.
Save article to Kindle
To save this article to your Kindle, first ensure [email protected] is added to your Approved Personal Document E-mail List under your Personal Document Settings on the Manage Your Content and Devices page of your Amazon account. Then enter the ‘name’ part of your Kindle email address below. Find out more about saving to your Kindle .
Note you can select to save to either the @free.kindle.com or @kindle.com variations. ‘@free.kindle.com’ emails are free but can only be saved to your device when it is connected to wi-fi. ‘@kindle.com’ emails can be delivered even when you are not connected to wi-fi, but note that service fees apply.
Find out more about the Kindle Personal Document Service.
- Volume 27, Issue 9
- Anna Björk (a1) , Ann-Marie Svensson (a2) , Mir Nabi Pirouzi Fard (a2) , Peter Eriksson (a1) and Mikael Dellborg (a1)
- DOI: https://doi.org/10.1017/S1047951117000968
Save article to Dropbox
To save this article to your Dropbox account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Dropbox account. Find out more about saving content to Dropbox .
Save article to Google Drive
To save this article to your Google Drive account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Google Drive account. Find out more about saving content to Google Drive .
Reply to: Submit a response
- No HTML tags allowed - Web page URLs will display as text only - Lines and paragraphs break automatically - Attachments, images or tables are not permitted
Your details
Your email address will be used in order to notify you when your comment has been reviewed by the moderator and in case the author(s) of the article or the moderator need to contact you directly.
You have entered the maximum number of contributors
Conflicting interests.
Please list any fees and grants from, employment by, consultancy for, shared ownership in or any close relationship with, at any time over the preceding 36 months, any organisation whose interests may be affected by the publication of the response. Please also list any non-financial associations or interests (personal, professional, political, institutional, religious or other) that a reasonable reader would want to know about in relation to the submitted work. This pertains to all the authors of the piece, their spouses or partners.
- Research article
- Open access
- Published: 21 February 2013
Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study
- Mora Murri 1 ,
- Isabel Leiva 2 ,
- Juan Miguel Gomez-Zumaquero 3 ,
- Francisco J Tinahones 4 , 7 ,
- Fernando Cardona 1 , 4 ,
- Federico Soriguer 5 , 6 &
- María Isabel Queipo-Ortuño 1 , 4
BMC Medicine volume 11 , Article number: 46 ( 2013 ) Cite this article
33k Accesses
30 Altmetric
Metrics details
A recent study using a rat model found significant differences at the time of diabetes onset in the bacterial communities responsible for type 1 diabetes modulation. We hypothesized that type 1 diabetes in humans could also be linked to a specific gut microbiota. Our aim was to quantify and evaluate the difference in the composition of gut microbiota between children with type 1 diabetes and healthy children and to determine the possible relationship of the gut microbiota of children with type 1 diabetes with the glycemic level.
A case-control study was carried out with 16 children with type 1 diabetes and 16 healthy children. The fecal bacteria composition was investigated by polymerase chain reaction-denaturing gradient gel electrophoresis and real-time quantitative polymerase chain reaction.
The mean similarity index was 47.39% for the healthy children and 37.56% for the children with diabetes, whereas the intergroup similarity index was 26.69%. In the children with diabetes, the bacterial number of Actinobacteria and Firmicutes, and the Firmicutes to Bacteroidetes ratio were all significantly decreased, with the quantity of Bacteroidetes significantly increased with respect to healthy children. At the genus level, we found a significant increase in the number of Clostridium, Bacteroides and Veillonella and a significant decrease in the number of Lactobacillus, Bifidobacterium, Blautia coccoides/Eubacterium rectale group and Prevotella in the children with diabetes. We also found that the number of Bifidobacterium and Lactobacillus , and the Firmicutes to Bacteroidetes ratio correlated negatively and significantly with the plasma glucose level while the quantity of Clostridium correlated positively and significantly with the plasma glucose level in the diabetes group.
Conclusions
This is the first study showing that type 1 diabetes is associated with compositional changes in gut microbiota. The significant differences in the number of Bifidobacterium, Lactobacillus and Clostridium and in the Firmicutes to Bacteroidetes ratio observed between the two groups could be related to the glycemic level in the group with diabetes. Moreover, the quantity of bacteria essential to maintain gut integrity was significantly lower in the children with diabetes than the healthy children. These findings could be useful for developing strategies to control the development of type 1 diabetes by modifying the gut microbiota.
Peer Review reports
Type 1 diabetes is a worldwide problem, mainly in children, and it is associated with a significant burden, mostly related to the development of vascular complications [ 1 ]. Type 1 diabetes is the result of a complex interaction between different degrees of genetic susceptibility and environmental factors [ 2 – 4 ]. The intestinal microbiota is one of these environmental factors currently under study, partly as a result of observations in both non-obese diabetic (NOD) mice and BioBreeding diabetes-prone rats, where the use of antibiotics was shown to prevent the onset of diabetes [ 5 , 6 ]. Moreover, a recent study using NOD mice suggested that the development of type 1 diabetes can be prevented through modulation of the intestinal microbiota [ 7 ]. Newly, Vaarala et al . suggested that the interaction between the intestinal environment, the barrier function and the immune system are crucial in the onset of type 1 diabetes [ 4 ]. Using a rat model, Roesch et al . found significant differences at the time of diabetes onset in the bacterial communities responsible for type 1 diabetes modulation [ 8 ]. Moreover, other studies have shown that beneficial bacteria, such as probiotic bacteria, have a protective effect in rodent models by delaying or preventing the onset of type 1 diabetes [ 9 , 10 ]. With respect to mechanisms of action, Wen et al . found that the gut microbiome of NOD mice lacking an adaptor for multiple innate immune receptors responsible for recognizing microbial stimuli correlates with the disease onset, revealing a relationship between gut microbiota and the immune system [ 11 ]. Recent studies have demonstrated that commensal bacteria are crucial for maturation and function of the mucosal immune system. The balance between two major effector T cell populations in the intestine, IL-17+ T helper 17 cells and Foxp3+ regulatory T cells, requires signals from commensal bacteria and is dependent on the composition of the intestinal microbiota [ 12 – 14 ]. In addition, increased gut permeability has been observed in patients with type 1 diabetes as well as in NOD mouse and BioBreeding rat models [ 15 – 18 ]. It has been suggested that this increased gut permeability (commonly called leaky gut) may affect the absorption of antigens that can attack and damage pancreatic beta cells [ 19 ]. Because gut microbes can affect intestinal permeability, the gut ecology may play a role in the development of type 1 diabetes [ 20 ].
Only a few studies have evaluated the ecology of intestinal microbiota in autoimmune children who were not yet diabetic [ 21 , 22 ]. These studies used a very low number of participants (four patients and four controls) and neither of them have controlled for such an important factor as the mode of delivery (natural birth or Cesarean) or the type and time of infant feeding (formula-fed or breast-fed), both of which determine the gut microbial composition during infancy [ 23 , 24 ].
The aim of the present study, therefore, was to characterize the composition of fecal microbiota in children with type 1 diabetes as compared with children without diabetes (controlling for such factors as mode of delivery and breastfeeding time) using PCR-denaturing gradient gel electrophoresis (DGGE) and real-time quantitative PCR (qPCR) analysis. This was to determine whether there were significant differences in the gut microbiota composition between these groups and, if so, to quantify the differences and determine the possible relation of the gut microbiota of children with type 1 diabetes with their glycemic level.
Study participants and design
The case-control study included 16 Caucasian children with type 1 diabetes, aged 7.16 ±0.72 years, and 16 healthy Caucasian children, aged 7.48 ±0.87 years. Type 1 diabetes was diagnosed following the criteria of the American Diabetes Association [ 25 , 26 ] and the appearance of at least two persistent, confirmed anti-islet autoantibodies (insulin autoantibodies, glutamic acid decarboxylase autoantibodies or tyrosine phosphatase autoantibodies). The patients with diabetes were treated and monitored according to a standard medical protocol. Patients were excluded if they had any other acute or chronic inflammatory diseases or infectious diseases at study entry. The study participants received no antibiotic treatment, probiotics, prebiotics or any other medical treatment influencing intestinal microbiota during the 3 months before the start of the study. The selected healthy children were all type 1 diabetes autoantibody negative and they were matched to the children with diabetes for age, gender, race, mode of delivery and duration of breastfeeding. The parents of the patients and controls completed a structured interview to obtain the following data: health status, lifestyle aspects (such as living environment and physical activity) and dietary habit. The dietary intake patterns in patients and controls were determined from a food frequency questionnaire that allowed us to assess the consumption of groups of foods. The written guardian or parental consents of the children were obtained. The sampling and experimental processes were performed with the approval of the local Ethics Committee of Ciudad de Jaen hospital. Stool samples were collected by parents at home and delivered to the storage area for frozen storage at -80ºC within one hour [ 27 ].
Anthropometric measurements
Body weight and height were measured according to standardized procedures [ 28 ].
Laboratory measurements
Fasting venous blood samples were collected. The serum was separated in aliquots and immediately frozen at -80ºC. Serum biochemical parameters were measured in duplicate. Serum glucose, cholesterol and triglycerides were measured using a standard enzymatic method (Randox Laboratories Ltd., Antrim, UK). The quantitative detection of autoantibodies to islet cell antigens was done using the Elisa RSR GADAb Kit, Elisa RSR IA-2Ab Kit and RIA RSR IAA Kit (RSR Limited, Cardiff, UK).
DNA extraction from fecal samples
Fecal samples were immediately kept after collection at -80°C and stored until analyzed. DNA extraction from 200 mg of stools was done using the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. The DNA concentration was determined by absorbance at 260 nm (A260), and the purity was estimated by determining the A260 to A280 ratio with a Nanodrop spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA).
Analysis of fecal microbiota by PCR-DGGE
Fecal samples from each participant were examined by determining PCR-DGGE profiles as recently published by us [ 29 ]. The V2 to V3 regions of the 16S rRNA genes (positions 339 to 539 in the Escherichia coli gene) of bacteria in the fecal samples were amplified by primers HDA1-GC (5′- CGC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCA CGG GGG G CC TAC GGG AGG CAG CAG T-3′; (the GC clamp is in boldface)) and HDA2 (5′-GTA TTA CCG CGG CTG CTG GCA C-3′) generating a 200 bp product. Aliquots (2 μL) of DNA were amplified by real-time PCR (20 μL final volume) in a 7500 Fast Real-Time PCR Systems instrument using Fast SYBR Green Master Mix and 200 nM of each of the universal primers HDA1-GC or HDA2 with the following amplification program: initial denaturation at 95°C for 20 s; amplification using 45 cycles including denaturation at 95°C for 3 s; annealing at 55°C for 30 s; and extension at 72°C for 1 min. Negative controls without a DNA template were included in each analysis.
After real-time PCR, 15 μL of products were mixed with 6 μL of loading dye before loading. Electrophoresis was performed with a DCode Universal Mutation Detection System instrument (Bio-Rad Laboratories, S.A, Madrid, Spain). Six percent polyacrylamide gels were prepared and electrophoresed with 1× TAE buffer prepared from 50× TAE buffer (2 M Tris base, 1 M glacial acetic acid, 50 mM ethylenediaminetetraacetic acid (EDTA)). The denaturing gradient was formed by using two 6% acrylamide (acrylamide to bisacrylamide ratio 37.5:1) stock solutions (Bio-Rad). The gels contained a 20% to 80% gradient of urea and formamide that increases in the direction of electrophoresis. Electrophoretic runs were in a TAE buffer (40 mmol/L Tris, 20 mmol/L acetic acid, and 1 mmol/L EDTA, pH 7.4) at 130 V and 60°C for 4.5 h. Electrophoresis was stopped when a xylene cyanol dye marker reached the bottom of a gel. Gels were stained with ethidium bromide (0.5 mg/L) for 5 min, rinsed with deionized water, viewed by UV transillumination and photographed with Gelcapture image acquisition software (DNR Bio-Imaging Systems Ltd, Mahale HaHamisha, Jerusalen, Israel). All the samples were analyzed on the same DGGE run to avoid the possible influence of variations in electrophoretic conditions between different runs. No band was observed in the negative controls. Similarities between banding patterns in the DGGE profile were calculated based on the presence and absence of bands and expressed as a similarity coefficient. Gels were analyzed using BioNumerics software (Applied Maths, Sint-Martens-Latem, Belgium). Normalized banding patterns were used for cluster analysis. The Dice similarity coefficient was used to calculate pairwise comparisons of the DGGE fingerprint profiles obtained. A similarity coefficient value of 100% indicates that DGGE profiles are identical while completely different profiles result in a similarity coefficient value of 0%. The unweighted pair group method with arithmetic mean algorithm was used for construction of dendrograms.
Sequencing of selected bands from DGGE gels
Bands were excised from DGGE gels with a sterile razor, placed in 40 μL sterile water and incubated at 4°C for diffusion of DNA into the water. DNA were used in a second PCR with HDA1/2 primers without a GC-clamp (initial denaturation at 95°C for 20 s, followed by 45 cycles including denaturation at 95°C for 3 s, annealing at 55°C for 15 s and extension at 72°C for 10 s). Subsequently, the PCR products were directly cloned into pCR 4-TOPO (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Plasmid DNA was isolated from the cells using the Qiagen Mini Spin Prep Kit (Qiagen), and subjected to PCR (HDA1/2-GC) as earlier described. PCR products were diluted until 20 ng/μL, purified with ExoSAP-IT (USB Corporation, Cleveland, OH, USA) and sequenced in an ABI 3130 (Applied Biosystems, Inc., Foster City, CA, USA) using the BigDie-Kit-Standard. Nucleotide sequence data obtained were analyzed using MicroSeqID v2.1.1 software (Applied Biosystems).
Microbial quantification by real-time qPCR
Specific primers targeting different bacterial genera were used to characterize the fecal microbiota by quantitative real-time qPCR (Table 1 ) [ 30 – 36 ]. Briefly, quantitative PCR experiments were performed with a LightCycler 2.0 PCR sequence detection system using the FastStart DNA Master SYBR Green kit (Roche Diagnostics, Indianapolis, IN, USA). All PCR tests were carried out in duplicate, with a final volume of 20 μL containing 1 μL of each fecal DNA preparation and 200 nM of each primer (Table 1 ). The thermal cycling conditions used were as follows: an initial DNA denaturation step at 95°C for 10 min; 45 cycles of denaturation at 95°C for 10 s; primer annealing at optimal temperature for 20 s; extension at 72°C for 15 s. Finally, melt curve analysis was performed by slowly cooling the PCRs from 95°C to 60°C (0.05°C per cycle) with simultaneous measurement of the SYBR Green I signal intensity. Melting-point-determination analysis allowed the confirmation of the specificity of the amplification products. Each participant's extracted DNA was subjected to a human β-globin PCR to ensure that amplifiable DNA was successfully extracted from the sample and to monitor for PCR inhibitors with the reaction conditions described previously [ 37 ]. The bacterial concentration from each sample was calculated by comparing the threshold cycle values obtained from the standard curves with the LightCycler 4.0 software. Standard curves were constructed for each experiment using serial 10-fold dilutions of bacterial genomic DNA (of known concentration) from pure cultures, corresponding to 10 1 to 10 10 copies per gram of feces.
The different strains used were obtained from the Spanish Collection of Type Cultures (CECT) ( Bacteroides vulgatus NCTC 11154, Fusobacterium varium NCTC 10560, Enterococcus faecalis CECT 184, Enterobacter cloacae CECT 194, Clostridium perfringens CECT 376) and the American Type Culture Collection (ATCC) ( Bifidobacterium bifidum ATCC 15696, Lactobacillus casei ATCC 334D-5, Prevotella intermedia ATCC 25611D-5, Ruminococcus productus ATCC 27340D-5 and Veillonella dispar ATCC 17745). Standard curves were normalized to the copy number of the 16S rRNA gene for each species. For species for which the copy number of 16S rRNA operon was not published, the copy number was calculated by averaging the operon numbers of the closest bacterial taxa from the ribosomal RNA database rrnDB [ 38 ]. Negative controls containing all the elements of the reaction mixture except template DNA were performed in every analysis and no product was ever detected. The data presented are the mean values of duplicate real-time qPCR analyses. The amplification efficiency of the qPCR for all primer pairs was determined using the linear regression slope of a dilution series based on the following equation E = 10 (-1/slope) . We found that for 13 primer pairs the efficiency ranged from 98% (E = 1.96) to 100% (E = 2) with slopes values in the range of -3.4 to -3.32.
Statistical analysis
Results are expressed as mean values and standard deviations. The statistical analysis was performed with SPSS 15.0 software (SPSS Inc., Chicago, IL, USA). The sample size was calculated to obtain a difference in the mean bacterial number between the healthy children and those with type 1 diabetes of at least 2 × 10 5 copies per gram of feces. With a power of 80%, an alpha error of 0.05 and an estimated standard deviation between group of 1.13 × 10 5 copies per gram of feces (data obtained from Wu et al . [ 39 ]), six children were needed in each group. However, we increased the number of participants to 16 children and 16 controls. The bacterial copy number values were converted into logarithmic values before the statistical analysis. Given the low number of participants analyzed, the Mann-Whitney U test was used to check changes in bacterial number and biochemical variables between the two groups. The Spearman correlation coefficient was calculated to estimate the linear correlations between variables. A multivariate regression analysis was performed to identify individual bacteria as independent predictors for plasma glucose level. Statistical significance was set at a P value of <0.05. All data are presented in the text as the mean ± SD.
The healthy children and those with diabetes all had similar physical activity and dietary habits. The analysis of the food frequency questionnaires showed no significant differences in the consumption patterns of rice, wheat, vegetables, fish or meat between the two study groups, although the children with diabetes had a fast carbohydrate restriction (foods made with white flour and refined sugar).
Anthropometric and biochemical measurements
The anthropometric and biochemical variables of the healthy children and those with diabetes are shown in Table 2 . Apart from the levels of glucose and HbA1c, which were significantly higher in the children with diabetes, no other significant differences were seen between the groups in the anthropometric and biochemical variables. In addition, because the healthy children and the children with diabetes were matched for breastfeeding time and mode of delivery, no significant differences were noted in these variables.
PCR-DGGE and bacterial band identification
Variations were found in the presence or absence (qualitative) and intensity (quantitative) of the bands between the healthy children and the children with diabetes in the host-specific fingerprints generated. DGGE band profiles showed differences in band richness between the two groups. Analysis of the diversity of the microbiota showed that the mean of the DGGE bands was 13.85 ±3.87 for the healthy children and 11.63 ±3.64 for the children with diabetes, though the difference was not significant. Some bands were seen in fingerprints from all the children (in different lanes but at the same position), indicating that specific species of the predominant microbiota were common to all the children.
The Dice similarity coefficient was used to calculate the similarity index between DGGE band profiles related to sampling of healthy children and those with diabetes. The mean similarity index was 47.39% for the healthy children and 37.56% for the children with diabetes. The mean similarity index between the groups was 26.69%, lower than the intra-group similarity (Table 3 ). The DGGE gel and the results of the cluster analysis are shown in Figure 1 . The cluster analysis showed that the intra-group similarity for the diabetic and the healthy groups was significantly higher than the inter-group similarity. These results demonstrate that the dominant microbiota in the healthy group was different from that of the diabetic group.
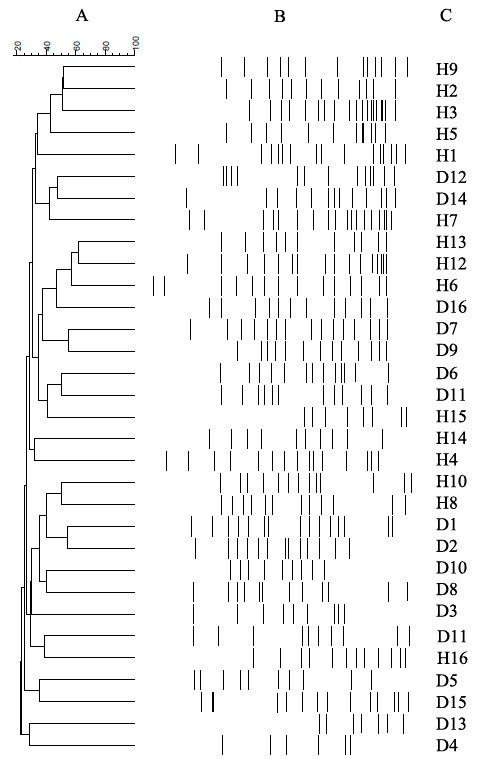
Cluster analysis . Dendrograms of electrophoretic band patterns obtained in the denaturing gel gradient electrophoresis experiment with universal primers in the fecal samples collected from healthy children (H) and those with type 1 diabetes (D). (A) Cluster analysis; (B) DGGE profiles related to fecal samples; (C) line graph.
All the bands from the profiles of all the healthy children and the children with diabetes were cloned and sequenced to identify the dominant microbiota. The sequence similarity matches for bands were analyzed by MicroSeqID v2.1.1 software. Bacterial identification showed that the majority of bacteria represented in the fingerprints obtained corresponded to five phyla (Table 4 ). Most of the sequences belonged to Firmicutes and Bacteroidetes, with the rest distributed among Actinobacteria, Fusobacteria and Proteobacteria. Nevertheless, we observed important differences between the healthy children and the children with diabetes in the distribution ratio of the different genera within Bacteroidetes, Firmicutes and Actinobacteria phyla. In the children with diabetes, we found an increase in the Clostridium, Bacteroides, Veillonella, Eggerthella and Bacillus frequencies and a disappearance of Prevotella and Bifidobacterium as compared with the healthy children (Table 4 ).
Comparative analysis of gut microbiota communities in healthy children and children with diabetes
Changes in the bacterial population abundance were assessed in the fecal samples of both groups. The results obtained in the real-time qPCR experiments with the different primers are shown in Tables 5 and 6 . Relevant differences were found in the bacteria number of three phyla between the diabetic and the healthy children. The number of Actinobacteria, Firmicutes and Bacteroidetes were significantly different between groups whilst the quantity of Proteobacteria and Fusobacteria were similar between the groups. In the children with diabetes, the bacterial number of Actinobacteria and Firmicutes was significantly decreased while that of Bacteroidetes was significantly increased with respect to the healthy children. Moreover, the Firmicutes to Bacteroidetes ratio was significantly lower in the children with diabetes than the healthy children.
Within Firmicutes, the quantity of Veillonella was significantly higher and the number of bacteria from the Blautia coccoides - Eubacterium rectale group was significantly lower in the children with diabetes compared with the healthy children. The Lactobacillus number was significantly lower and Clostridium levels significantly higher in the children with diabetes. However, no significant differences were found in Enterococcus levels between the two groups. Within Bacteroidetes, the quantity of Bacteroides was significantly higher whereas the number of Prevotella was significantly lower in the children with diabetes compared with the healthy children. Finally, within Actinobacteria, the number of Bifidobacterium was significantly lower in the children with diabetes.
Relationship between gut microbiota composition in children with type 1 diabetes and glycemic level
In the children with diabetes, we found a significant univariate correlation between the amount of specific bacterial groups and the plasma glucose levels ( Bifidobacterium r = -0.797, P = 0.008; Clostridium r = 0.676, P <0.05; Lactobacillus r = -0.698, P <0.05; and Firmicutes to Bacteroidetes ratio r = -0.473, P <0.05) and HbA1c levels ( Bifidobacterium r = -0.573, P <0.05; Clostridium r = 0.452, P <0.05; Firmicutes r = -0.559, P <0.05; Firmicutes to Bacteroidetes ratio r = -0.765, P = 0.012). A multivariate regression analysis that included all the bacterial groups analyzed showed that only the reduction in the number of Bifidobacterium and Lactobacillus was associated with the plasma glucose level ( P <0.05, β = -0.476, R 2 = 0.587; and P = 0.012, β = -0.687, R 2 = 0.539, respectively) whereas the higher HbA1c level was associated with the decrease in the Firmicutes to Bacteroidetes ratio ( P <0.001, β = -1.047, R 2 = 0.781) and the increase in the number of Clostridium ( P = 0.016, β = 0.867, R 2 = 0.499).
In the present study we found significant differences in the fecal microbial composition between healthy children and children with type 1 diabetes. We are unaware of any other similar studies in children with type 1 diabetes using simultaneously DGGE molecular profiling, unweighted pair group method with arithmetic mean algorithm dendrogram construction, sequencing and real-time qPCR analysis. To determine the characteristics of the gut microbiota based on the condition of just type 1 diabetes, we excluded the influence of physiological factors such as age, gender, dietary habits and race. In addition, we also controlled for the mode of delivery at birth and the duration of breastfeeding. This was because the first year of life has a crucial impact on gut microbiota composition and epidemiological studies in humans at genetic risk for type 1 diabetes have suggested that a short duration of breastfeeding and early feeding in infancy with complex dietary proteins such as cow's milk proteins can modulate the development of beta cell autoimmunity, clinical type 1 diabetes, or both [ 40 – 42 ]. No significant differences were found between the two groups of children (type 1 diabetes and controls).
The DGGE analysis of the fecal microbiota revealed a significantly lower intra-group similarity index in children with diabetes than in healthy children. In other words, the DGGE profiles in healthy children were more similar to each other, whereas in children with diabetes they were less similar. A similar result was found by Giongo et al . [ 21 ]. These data suggest that diabetic status may influence specific bacterial groups of the gut microbiota community.
Sequence analysis of the DGGE bands cloned enable the association of specific bacterial genotypes with health or diabetes situations. Consistent with previous human and animal studies [ 11 , 21 , 39 , 37 , 43 ], the gut microbiota of healthy children and children with diabetes was predominately composed of Firmicutes and Bacteroidetes and the main difference lies in the proportion of genus-division bacteria within this two phyla and the Actinobacteria phylum between both the group with diabetes and the healthy group. These results suggest that the dominant microbiota genera are different in children with type 1 diabetes compared with healthy children. Recently, three robust clusters, referred to as "enterotypes", which are not nation or continent specific have been identified. Assignment of an individual microbiome into a given enterotype is based upon the relative enrichment of that microbiome in one of three genera: Bacteroides (enterotype 1), Prevotella (enterotype 2) or Ruminococcus (enterotype 3) [ 44 ]. In this study, within Bacteroidetes, the Bacteroides genus was prevalent in the diabetic group, whereas the Prevotella genus was associated with the healthy group. Thus, the type 1 diabetic gut microbiomes could be classified into enterotype 1 and the healthy microbiomes could be classified into enterotype 2.
As DGGE is considered a semiquantitative tool for monitoring the dynamics of the predominant bacterial species of fecal microbiota, an additional analysis with real-time qPCR was performed to obtain a quantitative estimation of the changes found in the gut microbiota between children with diabetes and healthy children. We noted significant quantitative differences between the major microbial phyla present in the feces of healthy children and those with diabetes. In contrast to the situation in healthy children, we found a significant increase in the quantity of Bacteroidetes and a significant decrease in the number of Firmicutes and Actinobacteria in children with type 1 diabetes. Our data showed a significantly lower Firmicutes to Bacteroidetes ratio in children with type 1 diabetes compared with healthy children. Moreover, we saw a negative correlation between this ratio and both the glucose and the HbA1C levels in children with diabetes, which could help to explain the significantly higher glycemic level in this group. In agreement with this, Giongo et al . observed that the Firmicutes to Bacteroidetes ratio in study participants with type 1 diabetes was changing during the first 6 months after birth before the development of the autoimmune disease. These authors showed a successive decline in Firmicutes and an increase in Bacteroidetes number in the gut microbiome over time until the children became diabetic [ 21 ]. Moreover, this imbalance observed at the phylum level between Bacteroidetes and Firmicutes has been previously described in several human disorders. A decline in the Firmicutes to Bacteroidetes ratio compared with controls has been described in human type 2 diabetes [ 45 ], whereas in Crohn´s disease, both Bacteroidetes and Firmicutes seem to decline [ 46 ]. The opposite happens in obesity, where the imbalance is due to the increase in the Firmicutes to Bacteroidetes ratio [ 47 ], indicating that obesity and diabetes are associated with different groups of intestinal microbiota.
However, the major difference between the two groups was found in the number of bacteria at genus-division level. The most remarkable result was the significant increase in the number of Clostridium, Bacteroides and Veillonella in the children with diabetes, whereas the number of Lactobacillus, Bifidobacterium , the Blautia coccoides/Eubacterium rectale group and Prevotella genus were all significantly decreased in children with diabetes. Our findings concerning the microbiota of children with diabetes are in line with observations in other animal studies. Roesch et al . found higher levels of Lactobacillus and Bifidobacterium in BioBreeding diabetes-resistant rats whereas Bacteroides and Clostridium were more abundant in BioBreeding diabetes-prone rats [ 27 ]. In contrast with this, however, Brown et al . found that Lactobacillus and Bifidobacterium were more abundant in participants with type 1 diabetes than in healthy participants [ 22 ].
The significant decrease in the number of Lactobacillus and Bifidobacterium observed in children with type 1 diabetes in our study was associated with their higher levels of plasma glucose, as indicated by the negative correlation found. Also, the regression analysis showed that the decrease in the number of Lactobacillus and Bifidobacterium could be associated with the plasma glucose level in the children with diabetes. In previous studies, the levels of Bifidobacterium have also been related to improved glucose metabolism, insulin resistance and low-grade inflammation [ 48 , 49 ]. Moreover, Valladares et al . determined that the administration of Lactobacillus johnsonii isolated from BioBreeding diabetes-resistant rats delays or inhibits the onset of type 1 diabetes in BioBreeding diabetes-prone rats [ 10 ].
Both Lactobacillus and Bifidobacterium have members with probiotic characteristics and these have been associated with positive effects for the host in the large intestine [ 50 ]. In addition, both bacterial groups have the capacity to produce the beneficial organic acid lactate, which is converted into butyrate by butyrate-producing bacteria in the gut [ 22 ]. Barcenilla et al . [ 51 ] showed that most of the butyrate-producing isolates from human fecal samples are related to the Blautia coccoides-Eubacterium rectale group. Previous studies have shown that butyrate induces mucin synthesis (a glycoprotein produced by the host that could maintain the integrity of the gut epithelium) [ 52 ], decreases bacterial transport across the epithelium [ 53 ], and improves gut integrity by increasing tight junction assembly [ 54 ]. In addition, the genera Prevotella are responsible for the degradation of this mucin [ 55 ]; thus, the significant decline in the numbers of the Blautia coccoides-Eubacterium rectale group and Prevotella that we found in children with type 1 diabetes compared with healthy children could indicate a reduction in mucin synthesis by the host and a lack of this mucin on the epithelial layer of the gut, which would lead to a significant alteration in intestinal permeability. Other studies have described an association between type 1 diabetes and compromised barrier permeability in humans and both the NOD mouse and BioBreeding rat models [ 16 – 18 , 20 ].
The significant increase in the number of Clostridium, Bacteroides and Veillonella in the children with diabetes with respect to the healthy children was accompanied by a significant positive correlation between both the plasma levels of glucose and HbA1c and the quantity of Clostridium . These bacteria are able to ferment glucose and lactate to propionate, acetate and succinate. However, these short fatty acids do not induce mucin synthesis [ 52 ]. This situation would, though, reduce the tight junction assembly, generating an increase in the gut permeability in children with type 1 diabetes [ 22 ].
Finally, we propose a possible mechanism to explain the relationship we have found between the gut microbiota present in children with type 1 diabetes and the glycemic levels observed. The short-chain fatty acids (such as butyrate and propionate) formed by this gut microbiota have a role in the regulation of the levels of gut hormones such as glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1 and ghrelin. These hormones have important effects on carbohydrate metabolism [ 56 ], thus allowing gut microbiota to affect glycemic levels. In addition, Huml et al . have previously demonstrated an altered secretion pattern of gut hormones in children with type 1 diabetes that may impact on the metabolic control of diabetes in these patients [ 57 ]. Further studies will be necessary to demonstrate this proposed mechanism.
A limitation of the 16S rRNA gene-based method is that the function of the identified bacteria is unknown. Future studies using a microbial metagenomic sequencing analysis will be carried out to obtain information about the functional diversity of the bacterial community analyzed here.
This is the first study showing that type 1 diabetes is associated with compositional changes in gut microbiota. Our results show that gut microbiota found in children with type 1 diabetes differed significantly from that found in healthy children. The gut microbiota in the children with diabetes was less similar than the gut microbiota in the healthy children. The significant differences between the diabetic and the healthy children in the number of Bifidobacterium, Lactobacillus and Clostridium and the Firmicutes to Bacteroidetes ratio could be implicated in the glycemic level of the children with diabetes. In addition, the numbers of lactic acid-producing bacteria, butyrate-producing bacteria and mucin-degrading bacteria, essential to maintain gut integrity, were significantly lower in the children with diabetes than the healthy children. These bacterial differences could be responsible for the altered gut permeability previously described in patients with type 1 diabetes. These findings could be useful for developing strategies to control the development of type 1 diabetes by modifying the gut microbiota.
Abbreviations
denaturing gradient gel electrophoresis
ethylenediaminetetraacetic acid
non-obese diabetic mice
polymerase chain reaction
quantitative polymerase chain reaction
standard deviation
buffer with Tris base, glacial acetic acid and ethylenediaminetetraacetic acid.
Marcovecchio ML, Tossavainen PH, Dunger DB: Prevention and treatment of microvascular disease in childhood type 1 diabetes. Br Med Bull. 2010, 94: 145-164. 10.1093/bmb/ldp053.
Article CAS PubMed Google Scholar
Ehehalt S, Dietz K, Willasch AM, Neu A, Baden-Württemberg : Diabetes Incidence Registry (DIARY) Group. Epidemiological perspectives on type 1 diabetes in childhood and adolescence in Germany: 20 years of the Baden-Württemberg Diabetes Incidence Registry (DIARY). Diabetes Care. 2010, 3: 338-340.
Article Google Scholar
Patterson CC, Dahlquist G, Soltesz G, Green A, Grp EAS: Is childhood onset Type I diabetes a wealth-related disease? An ecological analysis of European incidence rates. Diabetologia. 2001, 4: 9-16.
Vaarala O, Atkinson MA, Neu J: The ''perfect storm'' for type 1 diabetes - the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes. 2008, 57: 2555-2562. 10.2337/db08-0331.
Article CAS PubMed PubMed Central Google Scholar
Brugman S, Klatter FA, Visser JT, Wildeboer-Veloo AC, Harmsen HJ, Rozing J, Bos NA: Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes?. Diabetologia. 2006, 49: 2105-2108. 10.1007/s00125-006-0334-0.
Schwartz RF, Neu J, Schatz D, Atkinson MA, Wasserfall C: Comment on: Brugman S et al. (2006) Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia 49:2105-2108. Diabetologia. 2007, 50: 220-221.
King C, Sarvetnick N: The incidence of type-1 diabetes in NOD mice is modulated by restricted flora not germ-free conditions. Plos One. 2011, 6: e17049-10.1371/journal.pone.0017049.
Roesch LFW, Lorca GL, Casella G, Giongo A, Naranjo A, Pionzio AM, Li N, Mai V, Wasserfall CH, Schatz D, Atkinson MA, Neu J, Triplett EW: Culture-independent identification of gut bacteria correlated with the onset of diabetes in a rat model. Isme J. 2009, 3: 536-548. 10.1038/ismej.2009.5.
Lai KK, Lorca GL, Gonzalez CF: Biochemical properties of two cinnamoyl esterases purified from a Lactobacillus johnsonii strain isolated from stool samples of diabetes-resistant rats. Appl Environ Microbiol. 2009, 75: 5018-5024. 10.1128/AEM.02837-08.
Valladares R, Sankar D, Li N, Williams E, Lai KK, Abdelgeliel AS, Gonzalez CF, Wasserfall CH, Larkin J, Schatz D, Atkinson MA, Triplett EW, Neu J, Lorca GL: Lactobacillus johnsonii N6.2 mitigates the development of type 1 diabetes in BB-DP rats. PLoS One. 2010, 5: e10507-10.1371/journal.pone.0010507.
Article PubMed PubMed Central Google Scholar
Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV: Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008, 45: 1109-1113.
Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR: Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009, 139: 485-498. 10.1016/j.cell.2009.09.033.
Ivanov II, Littman DR: Segmented filamentous bacteria take the stage. Mucosal Immunol. 2010, 3: 209-212. 10.1038/mi.2010.3.
Romano-Keeler J, Weitkamp JH, Moore DJ: Regulatory properties of the intestinal microbiome effecting the development and treatment of diabetes. Curr Opin Endocrinol Diabetes Obes. 2012, 19: 73-80.
Kuitunen M, Saukkonen T, Ilonen J, Akerblom HK, Savilahti E: Intestinal permeability to mannitol and lactulose in children with type 1 diabetes with the HLA-DQB1*02 allele. Autoimmunity. 2002, 35: 365-368. 10.1080/0891693021000008526.
Watts T, Berti I, Sapone A, Gerarduzzi T, Not T, Zielke R, Fasano A: Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc Natl Acad Sci. 2005, 102: 2916-2921. 10.1073/pnas.0500178102.
Bosi E, Molteni L, Radaelli MG, Folini L, Fermo I, Bazzigaluppi E, Piemonti L, Pastore MR, Paroni R: Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia. 2006, 49: 2824-2827. 10.1007/s00125-006-0465-3.
Lee AS, Gibson DL, Zhang Y, Sham HP, Vallance BA, Dutz JP: Gut barrier disruption by an enteric bacterial pathogen accelerates insulitis in NOD mice. Diabetologia. 2010, 53: 741-748. 10.1007/s00125-009-1626-y.
Vehik K, Dabelea D: The changing epidemiology of type 1 diabetes: why is it going through the roof?. Diabetes Metab Res Rev. 2011, 27: 3-13. 10.1002/dmrr.1141.
Article PubMed Google Scholar
Mathis D, Benoist C: The influence of the microbiota on type-1 diabetes: on the threshold of a leap forward in our understanding. Immunol Rev. 2012, 245: 239-249. 10.1111/j.1600-065X.2011.01084.x.
Giongo A, Gano KA, Crabb DB, Mukherjee N, Novelo LL, Casella G, Drew JC, Ilonen J, Knip M, Hyöty H, Veijola R, Simell T, Simell O, Neu J, Wasserfall CH, Schatz D, Atkinson MA, Triplett EW: Toward defining the autoimmune microbiome for type 1 diabetes. Isme J. 2011, 5: 82-91. 10.1038/ismej.2010.92.
Brown CT, Davis-Richardson AG, Giongo A, Gano KA, Crabb DB, Mukherjee N, Casella G, Drew JC, Ilonen J, Knip M, Hyöty H, Veijola R, Simell T, Simell O, Neu J, Wasserfall CH, Schatz D, Atkinson MA, Triplett EW: Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS One. 2011, 6: e25792-10.1371/journal.pone.0025792.
Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE: Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006, 118: 511-521. 10.1542/peds.2005-2824.
Musso G, Gambino R, Cassader M: Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded?. Diabetes Care. 2010, 3: 2277-2284.
Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997, 20: 1183-1197.
American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010, 3: S62.
Roesch LF, Casella G, Simell O, Krischer J, Wasserfall CH, Schatz D, Atkinson MA, Neu J, Triplett EW: Influence of sample storage on bacterial community diversity in fecal samples. Open Microbiol J. 2009, 3: 40-46. 10.2174/1874285800903010040.
Standardization of anthropometric measurements. The Airlie (VA) Consensus Conference. Edited by: Loham T, Roche A, Martorel R. 1988, Champaign, IL: Human Kinetics, 20-37.
Queipo-Ortuño MI, Boto-Ordóñez M, Murri M, Gomez-Zumaquero JM, Clemente-Postigo M, Estruch R, Cardona Diaz F, Andrés-Lacueva C, Tinahones FJ: Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am J Clin Nutr. 2012, 95: 1323-1334. 10.3945/ajcn.111.027847.
Guo X, Xia X, Tang R, Zhou J, Zhao H, Wang K: Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett Appl Microbiol. 2008, 47: 367-373. 10.1111/j.1472-765X.2008.02408.x.
Delroisse JM, Boulvin AL, Parmentier I, Dauphin RD, Vandenbol M, Portetelle D: Quantification of Bifidobacterium spp. and Lactobacillus spp. in rat fecal samples by real-time PCR. Microbiol Res. 2008, 163: 663-670. 10.1016/j.micres.2006.09.004.
Friswell MK, Gika H, Stratford IJ, Theodoridis G, Telfer B, Wilson ID, McBain AJ: Site and strain-specific variation in gut microbiota profiles and metabolism in experimental mice. PLoS One. 2010, 5: e8584-10.1371/journal.pone.0008584.
Stach JE, Maldonado LA, Ward AC, Goodfellow M, Bull AT: New primers for the class Actinobacteria: application to marine and terrestrial environments. Environ Microbiol. 2003, 5: 828-841. 10.1046/j.1462-2920.2003.00483.x.
Matsuki T, Watanabe K, Fujimoto J, Takada T, Tanaka R: Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl Environ Microbiol. 2004, 70: 7220-7228. 10.1128/AEM.70.12.7220-7228.2004.
Bekele AZ, Koike S, Kobayashi Y: Genetic diversity and diet specificity of ruminal Prevotella revealed by 16S rRNA gene-based analysis. FEMS Microbiol Lett. 2010, 305: 49-57. 10.1111/j.1574-6968.2010.01911.x.
Rinttilä T, Kassinen A, Malinen E, Krogius L, Palva A: Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J Appl Microbiol. 2004, 97: 1166-1177. 10.1111/j.1365-2672.2004.02409.x.
Fredricks DN, Fiedler TL, Thomas KK, Oakley BB, Marrazzo JM: Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. J Clin Microbiol. 2007, 45: 3270-3276. 10.1128/JCM.01272-07.
Lee ZMP, Bussema C, Schmidt TM: rrnDB: documenting the number of rRNA and tRNA genes in bacteria and archaea. Nucleic Acids Res. 2009, 37: 489-493. 10.1093/nar/gkn689.
Wu X, Ma C, Han L, Nawaz M, Gao F, Zhang X, Yu P, Zhao C, Li L, Zhou A, Wang J, Moore JE, Millar BC, Xu J: Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr Microbiol. 2010, 61: 69-78. 10.1007/s00284-010-9582-9.
Stuebe A: The risks of not breastfeeding for mothers and infants. Rev Obstet Gynecol. 2009, 2: 222-231.
PubMed PubMed Central Google Scholar
Vaarala O: The gut as a regulator of early inflammation in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2011, 18: 241-247. 10.1097/MED.0b013e3283488218.
Knip M, Virtanen SM, Becker D, Dupré J, Krischer JP, Åkerblom HK, TRIGR Study Group: Early feeding and risk of type 1 diabetes: experiences from the Trial to Reduce Insulin-dependent diabetes mellitus in the Genetically at Risk (TRIGR). Am J Clin Nutr. 2011, 94: 1814-1820. 10.3945/ajcn.110.000711.
Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE: Metagenomic analysis of the human distal gut microbiome. Science. 2006, 312: 1355-1359. 10.1126/science.1124234.
Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, et al: Enterotypes of the human gut microbiome. Nature. 2011, 473: 174-180. 10.1038/nature09944.
Larsen N, Vogensen FK, van den Beg FWL, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M: Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010, 5: e9085-10.1371/journal.pone.0009085.
Willing B, Halfvarson J, Dicksved J, Rosenquist M, Järnerot G, Engstrand L, Tysk C, Jansson JK: Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn's disease. Inflamm Bowel Dis. 2009, 15: 653-660. 10.1002/ibd.20783.
Armougom F, Henry M, Vialettes B, Raccah D, Raoult D: Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and methanogens in anorexic patients. PLoS One. 2009, 4: e7125-10.1371/journal.pone.0007125.
Philippe D, Favre L, Foata F, Adolfsson O, Perruisseau-Carrier G, Vidal K, Reuteler G, Dayer-Schneider J, Mueller C, Blum S: Bifidobacterium lactis attenuates onset of inflammation in a murine model of colitis. World J Gastroenterol. 2011, 17: 459-469. 10.3748/wjg.v17.i4.459.
Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM: Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009, 58: 1091-1103. 10.1136/gut.2008.165886.
Tzounis X, Rodriguez-Mateos A, Vulevic J, Gibson GR, Kwik-Uribe C, Spencer JP: Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am J Clin Nutr. 2011, 93: 62-72. 10.3945/ajcn.110.000075.
Barcenilla A, Pryde SE, Martin JC, Duncan SH, Stewart CS, Henderson C, Flint HJ: Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl Environ Microbiol. 2000, 6: 1654-1661.
Burger-van Paassen N, Vincent A, Puiman PJ, van der Sluis M, Bouma J, Boehm G, van Goudoever JB, van Seuningen I, Renes IB: The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J. 2009, 420: 211-219. 10.1042/BJ20082222.
Lewis K, Lutgendorff F, Phan V, Soderholm JD, Sherman PM, McKay DM: Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm Bowel Dis. 2010, 16: 1138-1148. 10.1002/ibd.21177.
Peng LY, Li Z, Green RS, Holzman IR, Lin J: Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009, 139: 1619-1625. 10.3945/jn.109.104638.
Wright DP, Knight CG, Parker SG, Christie DL, Roberton AM: Cloning of a mucin-desulfating sulfatase gene from Prevotella strain RS2 and its expression using a Bacteroides recombinant system. J Bacteriol. 2000, 182: 3002-3007. 10.1128/JB.182.11.3002-3007.2000.
Lin HV, Frassetto A, Kowalik EJ, Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G, Marsh DJ: Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012, 7: e35240-10.1371/journal.pone.0035240.
Huml M, Kobr J, Siala K, Varvařovská J, Pomahačová R, Karlíková M, Sýkora J: Gut peptide hormones and pediatric type 1 diabetes mellitus. Physiol Res. 2011, 60: 647-658.
CAS PubMed Google Scholar
Pre-publication history
The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1741-7015/11/46/prepub
Download references
Acknowledgements
The authors wish to thank all the participants for their collaboration and the Plataforma de Secuenciacion y Genotipado and the Unidad Central de Biología Molecular (Servicio de PCR a tiempo real) of FIMABIS for their help in laboratory assays. We also gratefully acknowledge the help of Ian Johnstone for his expertise in preparing this manuscript. The research group belongs to the Centros de Investigación en Red (CIBEROBN, CB06/03/0018 and CIBERDEM, CB07/08/0019) of the Instituto de Salud Carlos III. This work was partially funded by a grant from CIBER, CB06/03/0018 of the Instituto de Salud Carlos III to MM, FC, FJT and MIQO; the Instituto de Salud Carlos III, Madrid, Spain (CP07/0095) to FJT; and the Servicio Andaluz de Salud, Andalucía, Spain (PI0696/2010) to FJT. The funding agencies had no role in the design and performance of the study, the interpretation of the data or the writing of the manuscript.
Author information
Authors and affiliations.
Biomedical Research Laboratory, Virgen de la Victoria Hospital (FIMABIS), Campus de Teatinos s/n, Málaga, 29010, Spain
Mora Murri, Fernando Cardona & María Isabel Queipo-Ortuño
Pediatric Endocrinology Service, Carlos Haya Materno Infantil Hospital, Avenida Arroyo de los Angeles, Málaga, 29011, Spain
Isabel Leiva
Molecular Biology Laboratory, Civil Hospital (IMABIS foundation), Plaza Hospital Civil s/n, Málaga, 29009, Spain
Juan Miguel Gomez-Zumaquero
CIBER of Physiopathology of Obesity and Nutrition (CIBEROBN), Instituto de Salud Carlos III, C/ Sinesio Delgado nº 4, Madrid, 28029, Spain
Francisco J Tinahones, Fernando Cardona & María Isabel Queipo-Ortuño
Endocrinology and Nutrition Service, Carlos Haya Hospital, Plaza Hospital Civil s/n, Málaga, 29009, Spain
Federico Soriguer
CIBER of Diabetes and Metabolic Diseases (CIBERDEM), Instituto de Salud Carlos III, C/ Sinesio Delgado nº 4, Madrid, 28029, Spain
Endocrinology and Nutrition Service, Virgen de la Victoria Hospital, Campus de Teatinos s/n, Málaga, 29010, Spain
Francisco J Tinahones
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to María Isabel Queipo-Ortuño .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors' contributions
IL, FC, FS, FJT and MIQO conceived the study and developed the experimental design. IL, FS, FJT and MIQO were responsible for acquisition and selection of all samples utilized in this study. MM, IL, FC, JMGZ and MIQO performed all laboratory assays. MM, IL, JMGZ, FC and MIQO compiled the database and performed statistical analysis and data interpretation. MM, IL, JMGZ, FC, FS, FJT and MIQO wrote the paper. FS, FJT and MIQO provided critical revision. All the authors have read and approved the final manuscript.
Mora Murri, Isabel Leiva contributed equally to this work.

Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Rights and permissions.
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Murri, M., Leiva, I., Gomez-Zumaquero, J.M. et al. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Med 11 , 46 (2013). https://doi.org/10.1186/1741-7015-11-46
Download citation
Received : 15 August 2012
Accepted : 21 February 2013
Published : 21 February 2013
DOI : https://doi.org/10.1186/1741-7015-11-46
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- butyrate-producing bacteria
- glycemic level
- gut integrity
- gut microbiota
- gut permeability
- HbA1c level
- lactic acid-producing bacteria
- mode of delivery
- type 1 diabetes
BMC Medicine
ISSN: 1741-7015
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- DOI: 10.4236/OJEPI.2014.43018
- Corpus ID: 18619779
Reducing Participation Bias in Case-Control Studies: Type 1 Diabetes in Children and Stroke in Adults
- C. Keeble , Stuart Barber , +2 authors G. Law
- Published 5 August 2014
- Open Journal of Epidemiology
Tables from this paper

6 Citations
Investigating solutions to minimise participation bias in case-control studies, participation rates in epidemiology studies and surveys: a review 2007–2015, adaptation of chain event graphs for use with case-control studies in epidemiology, learning through chain event graphs: the role of maternal factors in childhood type 1 diabetes, choosing a method to reduce selection bias: a tool for researchers.
- Highly Influenced
Adaptations and examples 3 . 1 Adaptations for full analyses of case-control studies
25 references, non-participation and adjustment for bias in case-control studies of periodontitis., effect of reminders on mitigating participation bias in a case-control study.
- Highly Influential
RISK FACTORS FOR STROKE : A CASE CONTROL STUDY -
Antenatal risk factors for childhood diabetes mellitus; a case-control study of medical record data in yorkshire, uk, participation bias assessment in three high-impact journals, non-participation may bias the results of a psychiatric survey, selection bias and its implications for case-control studies: a case study of magnetic field exposure and childhood leukaemia., increased hospital length of stay attributable to clostridium difficile infection in patients with four co-morbidities: an analysis of hospital episode statistics in four european countries, the swedish personal identity number: possibilities and pitfalls in healthcare and medical research, database on danish population-based registers for public health and welfare research, related papers.
Showing 1 through 3 of 0 Related Papers
Use of machine learning to identify characteristics associated with severe hypoglycemia in older adults with type 1 diabetes: a post-hoc analysis of a case-control study
Add to collection, downloadable content.
- Affiliation: School of Medicine, Department of Surgery
- Affiliation: School of Medicine, Department of Medicine
- Other Affiliation: Department of Medicine, SUNY Upstate Medical University, Syracuse, New York, USA
- Other Affiliation: Department of Mathematics, Washington University in St Louis, St Louis, Missouri, USA
- Affiliation: Gillings School of Global Public Health, Department of Nutrition
- Download PDFPDF Download PDF + Supplemental DataPDF + Supplementary Material Epidemiology/Health services research Use of machine learning to identify characteristics associated with severe hypoglycemia in older adults with type 1 diabetes: a post-hoc analysis of a case–control study http://orcid.org/0000-0002-9905-4855Nikki L B Freeman1, Rashmi Muthukkumar2, Ruth S Weinstock3, M Victor Wickerhauser4, http://orcid.org/0000-0003-2701-101XAnna R Kahkoska5,6 Correspondence to Dr Nikki L B Freeman; [email protected] Abstract Introduction Severe hypoglycemia (SH) in older adults (OAs) with type 1 diabetes is associated with profound morbidity and mortality, yet its etiology can be complex and multifactorial. Enhanced tools to identify OAs who are at high risk for SH are needed. This study used machine learning to identify characteristics that distinguish those with and without recent SH, selecting from a range of demographic and clinical, behavioral and lifestyle, and neurocognitive characteristics, along with continuous glucose monitoring (CGM) measures. Research design and methods Data from a case–control study involving OAs recruited from the T1D Exchange Clinical Network were analyzed. The random forest machine learning algorithm was used to elucidate the characteristics associated with case versus control status and their relative importance. Models with successively rich characteristic sets were examined to systematically incorporate each domain of possible risk characteristics. Results Data from 191 OAs with type 1 diabetes (47.1% female, 92.1% non-Hispanic white) were analyzed. Across models, hypoglycemia unawareness was the top characteristic associated with SH history. For the model with the richest input data, the most important characteristics, in descending order, were hypoglycemia unawareness, hypoglycemia fear, coefficient of variation from CGM, % time blood glucose below 70 mg/dL, and trail making test B score. Conclusions Machine learning may augment risk stratification for OAs by identifying key characteristics associated with SH. Prospective studies are needed to identify the predictive performance of these risk characteristics.
- Blood Glucose
- Diabetes Mellitus, Type 1
- Case-Control Studies
- Diabetes Complications
- Hypoglycemia
- Blood Glucose Self-Monitoring
- https://doi.org/10.17615/xtc0-ed39
- https://doi.org/10.1136/bmjdrc-2023-003748
- Attribution-NonCommercial 4.0 International
- BMJ Open Diabetes Research & Care
- American Diabetes Association
- National Center for Advancing Translational Sciences
This work has no parents.
| Thumbnail | Title | Date Uploaded | Visibility | Actions |
|---|---|---|---|---|
| 2024-06-27 | Public |
Select type of work
Master's papers.
Deposit your masters paper, project or other capstone work. Theses will be sent to the CDR automatically via ProQuest and do not need to be deposited.
Scholarly Articles and Book Chapters
Deposit a peer-reviewed article or book chapter. If you would like to deposit a poster, presentation, conference paper or white paper, use the “Scholarly Works” deposit form.
Undergraduate Honors Theses
Deposit your senior honors thesis.
Scholarly Journal, Newsletter or Book
Deposit a complete issue of a scholarly journal, newsletter or book. If you would like to deposit an article or book chapter, use the “Scholarly Articles and Book Chapters” deposit option.
Deposit your dataset. Datasets may be associated with an article or deposited separately.
Deposit your 3D objects, audio, images or video.
Poster, Presentation, Protocol or Paper
Deposit scholarly works such as posters, presentations, research protocols, conference papers or white papers. If you would like to deposit a peer-reviewed article or book chapter, use the “Scholarly Articles and Book Chapters” deposit option.
Loading metrics
Open Access
Peer-reviewed
Research Article
Living with and managing type 1 diabetes in humanitarian settings: A qualitative synthesis of lived experience and stakeholder tacit knowledge
Roles Data curation, Formal analysis, Writing – original draft
Affiliation MSc Public Health Graduate Class of 2023, London School of Hygiene & Tropical Medicine, London, United Kingdom
Roles Writing – review & editing
Affiliations International Committee of the Red Cross, Beirut Delegation, Lebanon, INSPECT-LB (Institut de Santé Publique, Epidemiologie Clinique et Toxicologie-Liban), Beirut, Lebanon
Roles Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation NCD in Humanitarian Settings Research Group and Centre for Global Chronic Conditions, London School of Hygiene & Tropical Medicine, London, United Kingdom
- Oria James,
- Linda Abbou-Abbas,
- Lavanya Vijayasingham

- Published: June 21, 2024
- https://doi.org/10.1371/journal.pgph.0003027
- Peer Review
- Reader Comments
Humanitarian health actors are beginning to better consider and manage non-communicable diseases, such as diabetes, in emergency and protracted crisis settings. However, a focus on the more globally prevalent type 2 diabetes (T2D) dominates. Blind spots prevail in the unmet needs for type 1 diabetes (T1D), a chronic autoimmune condition where individuals are unable to produce insulin, thereby dependent on lifelong insulin therapy and blood glucose management. Although some T1D management requirements overlap with those of T2D, the immediate risk of fatal complications following insulin therapy disruption, the earlier age of onset during childhood, adolescence or young adulthood, and its lower prevalence compared to T2D within communities and local health systems mean that T1D requires nuanced consideration and targeted interventions. Intending to inform program and policy design for people with T1D (PWT1D), we synthesized themes of lived experience from PLWT1D and their caregivers, and the tacit working knowledge of health providers and policymakers in the context of local humanitarian operations. Through a strategic search of health databases (up to July 2023), we identified 11 articles that include interview excerpts from PWT1D, caregivers, healthcare providers and policymakers about T1D management in humanitarian settings. We used reflexive thematic analysis to guide data extraction, coding, and synthesis, resulting in the identification of four overarching themes: food and insulin security, family relations, knowledge translation, and response to diagnosis. The narratives highlight harsh trade-offs made by PWT1D and their families in the face of insulin and food insecurity, as well as the damaging impact of low T1D education in families, communities and health systems. Targeted family and community-based solutions are urgently required, alongside systemic reforms and international collaboration to enable better T1D coping and management in humanitarian settings.
Citation: James O, Abbou-Abbas L, Vijayasingham L (2024) Living with and managing type 1 diabetes in humanitarian settings: A qualitative synthesis of lived experience and stakeholder tacit knowledge. PLOS Glob Public Health 4(6): e0003027. https://doi.org/10.1371/journal.pgph.0003027
Editor: Julia Robinson, PLOS: Public Library of Science, UNITED STATES
Received: February 27, 2024; Accepted: May 28, 2024; Published: June 21, 2024
Copyright: © 2024 James et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All data are in the manuscript and/or supporting information files.
Funding: The authors received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exists.
Introduction
In 2022, about 275 million people globally were estimated to need assistance as a result of a humanitarian crisis–increasing from 235 million in 2021 [ 1 ]. A humanitarian crisis or emergency refers to a singular or a series of events in a country or region, that causes serious disruption to the functioning of a society, resulting in human, material, or environmental losses which exceed the ability of affected people to cope using their own resources [ 2 ]. These crises are diverse, and can be categorised by its speed of onset (sudden or slow), its length (protracted) or cause (natural or man-made hazard or armed conflict, [ 2 , 3 ]. Ultimately, these dynamics tend to present a critical threat to health and well-being of the affected populations.
Humanitarian health operations have only recently begun to better manage non-communicable diseases such as heart disease and diabetes–a group of conditions that has been mostly ‘forgotten’ in past humanitarian operations [ 4 – 6 ]. The burden of diabetes morbidity and mortality is high–in over half of humanitarian settings, diabetes prevalence is more than 10%, and in some international non-governmental organization (NGO) supported physical rehabilitation centres, diabetes causes more than 25% of lower limb amputations [ 7 ]. Indeed, humanitarian crises and settings whether acute or protracted, are typically characterised by complex, disrupted and fast-changing dynamics, that physically, logistically, financially, and politically impede governments and other non-state actors from providing sufficient and continuous care to the population. National health systems often have limited local government funding, and more recently, receive lower levels of international donor funding [ 8 , 9 ]. The local health workforce tends to be under-capacitated and face high turnovers [ 9 ]. Logistical and supply chain limitations affect the availability of basic diagnostic equipment such as blood glucose monitors and life-saving therapies such as insulin [ 5 ]. The populations requiring care for NCDs often encounter significant financial obstacles when accessing private healthcare services. These challenges include out-of-pocket expenses for medical treatment and additional indirect costs such as transportation. Furthermore, individuals may also experience a loss of income due to the need to take time off work when seeking care-particularly in the context of daily wage employment, where they may be deterred from health-seeking even through public or subsidized healthcare delivery programs or schemes. [ 9 – 12 ].
Amidst these contextual barriers local health systems and humanitarian operations tend to place a stronger focus on the more prevalent type of diabetes—type 2 diabetes (T2D). Type 1 diabetes (T1D), also sometimes known as juvenile diabetes or insulin-dependent diabetes mellitus, is a chronic autoimmune disease. Nearly 9 million people are estimated to live with T1D globally- but T1D is also known to be grossly underdiagnosed and understudied in many world regions due to strained health and research infrastructure [ 13 , 14 ]. The main difference between T1D and T2D is that in the former, the body has no ability to produce insulin, and in the latter, the body has a reduced ability to produce sufficient insulin. In T1D, the beta cells in the pancreas are destroyed by an autoimmune assault, often thought to be a consequence of viral infection, environmental toxins, or genetic predisposition [ 15 ]. Destruction of beta cells results in the body’s inability to produce insulin, making the body incapable of blood glucose regulation, leading to glycaemic variability (GV) [ 16 ]. In T2D, the pancreas either does not produce enough insulin, or the body has become resistant to insulin [ 17 ]. Insulin resistance in T2D also leads to GV, but in milder or less advanced stages, T2D can present with less volatile and less life-threatening GV, compared to that in T1D [ 18 ]. Thus, while some people living with T2D may be able to manage their condition with oral medication and health behaviour changes, the only way to manage T1D is through the use of insulin therapy [ 19 , 20 ]. Without insulin, T1D always leads to death (Beran et al., 2016; Willner et al., 2020). Furthermore, unlike T2D, there is no known way to prevent T1D. This is based on current understandings of T1D aetiology which includes a complex combination of viral and environmental triggers, as well as phenotype and genetic differences that influence individual risk [ 13 ].
Due to the nature of the condition outlined above, all PWT1D require insulin to survive. Insulin deprivation is the most prevalent cause of death for children with T1D in low-income countries (LIC) [ 21 ]. In 2021, the average life expectancy of a ten-year-old diagnosed with T1D was 13 years in LICs, compared to 65 years in high-income countries [ 14 ]. While there are some variations in presentation and age of onset across different geographical regions, in low-and middle-income countries (LMIC), where most humanitarian operations reside, there are approximately 1.2 million people with T1D (PWT1D), with more than 50% under the age of 15 years old [ 13 , 22 , 23 ]. In humanitarian settings, where access to high quality and timely healthcare, diagnostic resources, and medication, is often fragmented and unaffordable, PLWT1D face many barriers to living long, productive, and disability-free lives.
Additionally, researchers have also hypothesized that patients with lower or non-existent insulin secretory capacity (e.g., T1D) are more sensitive to stressful environments (including emergency events in humanitarian settings and circumstances) than those with some insulin production capacity (e.g., T2D) [ 24 ]. Distressing life events, including injury and health-related events (e.g. severe accidents, death of a family member), are also known to be associated with an increased risk of developing T1D [ 25 ]. Population-based prospective and retrospective studies have shown that stressful life events (e.g. war) during the first 14 years of life may pose a particular risk for developing T1D [ 26 , 27 ]. After the 2004 Marmara Earthquake in Turkey, the average HbA1c of 88 PWT1D increased from 7.4 pre-earthquake to 8.5 three months after the earthquake [ 28 ]. Twelve months before and after the 2007 flooding of Hull in the United Kingdom, 60 PWT1D experienced an average HbA1c increase of 8.1 to 8.6 [ 29 ]. Similarly, after the 2011 Great East Japan Earthquake, the average HbA1c of 55 PWT1D rose from 7.8 pre-earthquake to 8.1 three months after the earthquake [ 24 ]. Notably, those with T2D did not experience any difference in HbA1c after the Great East Japan Earthquake (7.3 vs. 7.3), which could imply that PWT1D in disaster settings have increased glycaemic vulnerability compared to people with T2D.
However, despite these differences in need and risk, there is still a paucity of research and guidelines that serve PWT1D in humanitarian settings [ 30 ]. In the World Health Organisation’s (WHO) Package of Essential Noncommunicable Disease Interventions for Primary Health Care (WHO PEN), there is only one condition-specific recommendation for T1D, “Self-monitoring and self-adjustment of dosage is recommended in type 1 diabetes according to an agreed action plan with a health professional” (p. 57) [ 31 ] where service delivery may be compromised in an emergency or humanitarian setting. None of the targets, indicators, or policy options provided in the WHO GAP are specifically oriented towards T1D management. More encouragingly in humanitarian, emergency and resource-limited settings, a package of essential NCD interventions for humanitarian settings (PEN-H), developed by the International Rescue Committee (IRC) and the USAID, outlines the clinical management of T1D [ 32 ]. Similarly, the UN United Nations High Commissioner for Refugees (The UN Refugee Agency-UNHCR), IRC, and Informal Inter-Agency Group on NCDs in Humanitarian Settings outlines PWT1D as those at ‘immediate risk’ if there is interruption to care or when insulin access is compromised [ 33 ].
These guidelines and references focus on the clinical management, but do not include many discussions or consideration of lived experience of illness management or coping in people’s local context. The lived experience of PWT1D, and their caregivers, as well as the tacit work knowledge of medical professionals and policymakers can inform the development of more comprehensive guidelines, programs and policies aimed at promoting health and reducing health complications of PWT1D in emergency and humanitarian settings. Based on our assessment of literature, most studies about the lived experiences of PWT1D have either been conducted in high-income countries, or in stable settings within low- and-middle-income countries. Evidence about T1D in humanitarian settings is in its infancy, and tends to focus on the biomedical, quantifiable aspect of diabetes [ 30 ]. Little research addresses how T1D manifests or progresses during crises. Even less research examines the unique challenges of T1D management in crisis-affected settings where PWT1D have little control over their diet and access to essential medications and supplies. While a modest number of papers have assessed HbA1c changes and medication adherence in people with diabetes in humanitarian crises, to the best of our knowledge, it appears that no reviews have been published on the lived experiences of PWT1D to date.
This paper is a review and synthesis of the unique experiences of people living with and managing T1D amidst the contextual realities of humanitarian crises, with an aim to support policy development and program implementation. While highlighting the lived experiences of individuals and caregivers, we also include perspectives from healthcare providers and policymakers to provide a broader view of the structural factors that influence health-seeking behaviours and engagement with health resources.
We conducted a qualitative synthesis of lived experience of PWT1D and their caregivers, as well as the tacit working knowledge of managing T1D in humanitarian settings. Using a reflexive thematic analysis (RTA) [ 34 ], we viewed and analysed the data from a constructivist paradigm, with a subjectivist epistemology, a relativist ontology, and an interpretive methodology [ 35 ]. The reflexive approach features our active roles, as researchers, in the knowledge production. RTA is considered a reflection of researchers’ interpretive analysis at the intersection of 1) the data; 2) the theoretical assumptions underlying the analysis; and 3) the resources and skills of the researchers [ 34 ]. As such, in this approach, our subjective experience of living with T1D (author 1) and other chronic illnesses (author 3), as well as working on humanitarian programs and research on NCDs(authors 2 & 3) guide our engagement with the retrieved narratives and themes. We detail our positionality and reflexivity statement in a sub section below.
Conceptual background
Narratives of lived experience of illness, caregiving, and tacit knowledge of health care stakeholders within the social context
For individuals living with a chronic illness, management and coping abilities are context dependent. Experiences evolve over time and life course, with narratives that often contain themes of disruption, diminishment, and discontinuous coping, due to a lack of access to coping resources [ 36 – 39 ] Illness caregiving, especially amidst gaps in health and social care, is largely unpaid and informal, mostly delivered by family and friends, and often viewed as a moral choice or imperative [ 40 , 41 ].
The tacit knowledge from medical professionals, social care providers, and policy makers reveal the embedded, every-day knowledge acquired from working within a particular context, making the information explicit and known [ 42 , 43 ]. Overall, an amalgamation of personal biomedical, popular, socio-cultural, religious, moral, economic, and political influences shape the meanings and value judgements attributed to illness, and consequently the responsive action at individual, population, and systems levels [ 44 – 46 ].
T1D care challenges in humanitarian crises: a social determinants and health systems building blocks perspective
As outlined by the WHO, ‘health is determined by the conditions in which people are born, grow, work, live, and age, and the wider set of forces and systems shaping the conditions of daily life- the social determinants of health (SDoH) [ 47 ]. Under the Healthy People 2030 framework, SDoH are categorized into five different components: 1) Education access and quality; 2) Economic Stability; 3) Neighbourhood and Built Environment; 4) Social and community context; and 5) Health care access and quality [ 48 ]. Considering the aetiology of T1D, and applying the SDoH framework as a analytic lens, we explore how individual ability to manage and cope with T1D (e.g., administering insulin and adhering to a healthy diet) is influenced by a variety of ecological factors such as social and community contexts (e.g., gender norms), economic stability (e.g., poverty) and healthcare access and quality (e.g., access to providers trained in T1D care).
From a health access perspective, effective management of T1D is contingent on continuity of care within the local health system and ecosystem [ 49 ]. The WHO’s six building blocks of health systems framework was used as a second analytical framework to help guide the synthesis of qualitative data [ 50 , 51 ]. The framework describes the health system in terms of the six following components: 1) Service delivery; 2) Health workforce; 3) Health information systems; 4) Access to essential medicines; 5) Financing; and 6) Leadership and governance. Together, the SDoH and the WHO’s six building blocks of health systems frameworks helped guide the analysis process.
Deep engagement and critical reflections on the multiple and accumulative influences of the social and lived context is necessary to fully understand how and why illness produces different sets of consequences. Cumulatively, economic, political, and climate instability within humanitarian settings impact all domains of T1D care. First, health infrastructure such as prescription, pharmaceutical and delivery services may be disrupted, resulting in the rationing of insulin and other diabetes supplies (e.g., blood glucose meters, insulin needles) [ 52 ]. Rationing of insulin and glucose test supplies has also been reported in several countries with more stable health systems, where health financing and access schemes do not ensure an affordable, adequate or continuous supply (USA, Panama, India, and Canada) [ 53 ]. Disruption of food and potable water is also a significant danger to PWT1D, who are at risk of life-threatening GV without consistent, balanced meals [ 54 ]. Climate disasters, political instability, and military conflict also often result in the reallocation of people and resources, leading to overburdened or abandoned health systems impacting the availability and quality of diabetes care [ 55 ].
Data sources & search strategy
To identify studies reporting on the views and experiences of people with T1D, a search strategy for qualitative and mixed-methods articles was developed. The electronic search was run in five databases: Medline, Embase, Web of Science, Scopus, and PsychINFO. Databases were searched from their inception to July 2023, and search terms were related to T1D and humanitarian settings. After considerable consultation with a (masked) university librarian, a search strategy was crafted for Medline and modified for other databases [Supplementary Information 1].
Inclusion criteria for publications included:
- Published in peer-reviewed journals;
- Containing qualitative data and interview quotes;
- In English with no year restrictions;
- That reported on the lived experiences of PWT1D, their family members, caregivers, or healthcare providers; and
- Were based in a humanitarian setting impacted by international or domestic conflict, economic or political instability, infectious disease outbreaks, refugee crises or climate disasters. Articles that included qualitative data from people with T1D and T2D, or did not differentiate between T1D and T2D, were also included and evaluated.
Researcher reflexivity
Reflexivity refers to how the researcher and research process can shape the data collection, evidence synthesis, and conclusions drawn [ 56 ]. In line with the constructionist paradigm used that emphasizes the role of researcher subjectivity, we offer some reflections on how our own personal and professional histories inform our subjective interpretation and analytic lens.
(Author 1) is the primary researcher in this article, which was conducted as a part of her MSc in Public Health at (masked). The following is her reflexivity statement:
I am a young , Canadian woman who has lived with T1D since the age of ten . I have always had access to insulin and diabetes supplies through publicly funded healthcare systems in Canada and the United Kingdom . My lived experience with T1D has been one of privilege; I have never experienced food insecurity , had to ration my insulin , or been personally impacted by humanitarian crises . I have also been the grateful recipient of extensive diabetes management training , both through hospital programs as a patient , and of my own volition as a diabetes researcher . For many years , I have shared my experiences living with T1D on social media . I cherish the online diabetes community—they have provided me with non-judgemental support and insight that has transcended languages and health systems . Social media has opened my eyes to the many , diverse , experiences of PWT1D accepting their diagnosis , navigating health systems , accessing insulin and medical technology , and integrating diabetes management into their lives . Many of their experiences contrast my own experiences . On one hand , I am an insider , intimately aware of the physical and mental burdens of living with and managing T1D . On the other hand , I am an outsider , having only experienced T1D in settings characterized by stability and access . I am mindful that the lived experiences of PWT1D in humanitarian settings are very different from my circumstances–I read their interview excerpts with humility and recognition of my position . My research lens thus stems both from my personal experiences as a PWT1D and my professional experiences as a public health researcher .
Author 2 works for a humanitarian organization and is involved in humanitarian NCD programming in Lebanon. Author 3 supervised the MSc Public Health research project, providing insights from ongoing research on NCDs in humanitarian settings and her prior work on chronic illness lived experiences, which similarly drew on her personal experience of living with and researching another chronic autoimmune illness in a middle-income country.
Data extraction and synthesis.
We extracted the following material from all the included studies: year of publication, summary of the study’s aim, country of study, study context, methods of evaluation (e.g., semi-structured interviews), study population (e.g., PWT1D, family member) [ Table 1 ], and quotes about participants’ experience of T1D in humanitarian settings.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pgph.0003027.t001
We found that a reflexive thematic analysis (RTA) [ 34 ] was well-suited to achieve our research aims: to highlight the lived experiences of PWT1D and their caregivers, and the tacit knowledge of health stakeholders in humanitarian settings [ 57 ]. RTA is an interpretive approach, within a constructionist paradigm. In the RTA analysis process, our subjectivity as researchers is understood to be integral aspect of the analysis process. In RTA, themes are not deductive or predetermined. Instead, inductive code and themes are sought, and then organized around “central organization concepts” that are identified from the data [ 34 ]. The conceptual frameworks were only used to provide a broad gaze on social, contextual and health-system related factors that contribute to lived experiences.
We applied Braun and Clarke’s 6-step RTA process in the analysis: 1) Become familiar with the data, 2) Generate initial codes, 3) Construct themes, 4) Review potential themes, 5) Define and name themes, and 6) Write-up [ 58 ]. After familiarization with the literature, qualitative data from all included studies was aggregated into one document, printed out, and cut into individual quotes [Supplementary Information 2]. Initial codes were generated on a line-by-line basis using a pen and paper. After the articles underwent the initial stage of coding, additional re-reads were conducted, and updated codes were collated into candidate themes. Through ongoing reflection, the code groupings were amended until a smaller set of themes and subthemes remained. After data saturation was reached, themes and subthemes were established.
Search results
The database searches resulted in 912 publications, from which 184 duplicates were removed. The remaining 732 articles were then screened by abstracts, and 93 articles were selected for full-text review. References from these articles were reviewed, and four additional studies were added. After a more thorough review, 11 studies that met the inclusion criteria were identified and chosen for RTA. A flow chart of the literature search process is presented in Fig 1 .
https://doi.org/10.1371/journal.pgph.0003027.g001
Study characteristics
Of the 11 studies identified, six studies were conducted in Asia (55%), four in Africa (36%), and one in Europe (9%). Qualitative data collected from ten different countries: Syria, Tajikistan, Lebanon, Palestine, Japan, Ethiopia, the Democratic Republic of Congo, Liberia, Uganda, and Belgium. Study contexts included civil war (27%), ongoing conflict (27%), refugee and asylum settings (27%), COVID-19 (9%), and one hypothetical pre-disaster setting (9%). Two study designs were included, qualitative studies (8, 82%) and mixed-methods studies (2, 18%). Just under half the studies (5, 45%) exclusively included the views of PWT1D, while the others (6, 55%) contained views from both PWT1D and PWT2D, or included people with diabetes mellitus (DM) with no distinction in type provided. All studies were published in 2014 or later.
Quality appraisal
We used the consolidated criteria for reporting qualitative studies (COREQ) checklist [ 70 ] to assess the quality and transparency of the included studies. The COREQ Checklist is structured into three distinct domains, 1) Research team and reflexivity; 2) Study design; and 3) Analysis and findings. The assessment of these items aimed to provide insights into the potential biases and rigor of the included studies, without serving as exclusion criteria.
Domain 1: characteristics of the research team and their relationship with the study participants.
Most studies clearly indicated which researchers conducted the interviews or focus groups, and about half of them outlined the researcher’s expertise by describing their experiences or training. The studies provided moderate but adequate descriptions of the researchers’ personal characteristics. Describing the relationship with the participants, however, was a low priority among nearly all the studies. Few studies mentioned when relationships with the participants were established, or whether participants were informed about the goals of the researchers. As such, it is not possible to evaluate the nature or dynamics of the researcher-participant interactions, and whether important factors such as power dynamics or conflicts of interest may have impacted data collection. Further, none of the studies contained reflexivity statements from the researchers. Transparency about how researchers’ experiences and assumptions may have influenced the research process and findings was not prioritized.
Domain 2: study design and methodology
All studies described a clear methodological orientation, as well as a sampling strategy, sample size, data collection setting, and method of approach. However, one common gap among the included studies was information about non-participation. Few studies mentioned non-participation rates, and none of them explored the reasons for non-participation in detail. As a result, there is a reduced capacity to identify potential biases and assess the generalizability of the findings.
Domain 3: data analysis and findings.
Across all studies, description of the data analysis process and derivation of themes was clear. There were no identifiable gaps regarding the clarity and rigour of data coding. Notably, one commonality among the studies was the absence of information regarding participant feedback. Either this step was not deemed important to describe, was not able to occur, or did not occur. Participant feedback is an important step that improves credibility of the data and addresses potential biases. It also enriches the research findings by strengthening trust and rapport with the participants while validating their voices. The absence of this information could lead to a misrepresentation of participant experiences, potentially compromising the credibility and validity of study findings. Finally, reporting processes were strong. All studies presented consistency between the data and the findings, as well as clarity in major and minor themes.
Synthesis of qualitative studies
Four distinct, but related, themes were identified from the thematic analysis: 1) Food and insulin security, 2) Family caregiving, 3) Knowledge translation, and 4) Response to diagnosis. Themes with illustrative quotes are presented in Fig 2 .
https://doi.org/10.1371/journal.pgph.0003027.g002
Theme 1: Food and Insulin Security
“I could only come to pick supplies when [my parents] had money for me . There was a time I ran out of insulin even when I had tried to use it very sparingly , after realizing my parents had not got money for me … ” PWT1D [ 68 ]
Insulin insecurity was one of the most prominent themes identified in this review. In several studies, patients reported rationing their insulin intake to make their supply last longer; [ 59 , 68 ] a dangerous practice that can result in disability and death. Unfortunately, the phenomenon of ‘ rationing insulin’ is widely reported across LMICs where insulin can be unavailable, unaffordable, inaccessible, or rendered ineffective due to hermos-instability [ 52 ]. At times, insulin was so out of reach for patients that providers told PWT1D that they were likely going to die.
“If you have patients buying their own insulin , especially with the economy we are faced with in this country , it’s going to be difficult telling most patients there is nothing we can do but just to allow you to die . ” Healthcare provider [ 59 ]
Even when PWT1D could afford insulin, transportation posed another significant barrier to access. During the COVID-19 lockdown in Uganda, vehicle restrictions meant that trips to replenish insulin had to be done by foot, or by expensive hired vehicles.
“With the initial total lockdown , there were no means to travel but foot… . After easing some travel restrictions , the fares have doubled , and you must compete like never before . ” PWT1D [ 68 ]
Trips seeking health services could be dangerous. At times, they required payments to corrupt local enforcement officers.
“You had to deal with LDU [COVID-19 guidelines enforcement] . You would have to plead with them or get them something for your passage [corrupt through your way through] … . ” PWT1D [ 68 ]
Importantly, even when insulin was available in humanitarian settings, its sensitivity to temperature could render it useless in situations of prolonged heat or cold.
“… the only negative thing was the insulin in Syria . The electricity was really weak , and the insulin was not in the fridge . ” PWT1D [ 63 ]
Although emerging research has shown that insulin is more heat-stable than previously thought, cold storage still remains a barrier for many PWT1D in low resource settings [ 71 – 73 ]. In one article, only 30% of the PWT1D interviewed reported having access to a refrigerator for storage, and even then, spoke of problems with access to electricity [ 59 ]. PWT1D used to context- adapted alternative ways of keeping their insulin cool, such as storing their insulin coolers in banana trees by cutting small openings in the trunks [ 59 ]. Indeed, in the absence of refrigeration, adaptations such as the use of clay-pots and other evaporative cooling techeniques in low-resource settings have been found to be effective as a cooling strategy for insulin storage [ 72 , 74 ].
Interview transcripts highlighted the intimate relationship between access to insulin and access to food. The lived experiences of insulin insecurity could not be divorced from experiences of food insecurity.
“People who inject , they need to eat; they need to have certainty of meals . When they get home they worry , thinking they may die due to lack of food… You get discouraged because you have medicines but not the food … ” –PWT1D [ 66 ]
When PWT1D have access to insulin but experience food insecurity, GV is a major concern [ 75 ]. If insulin is taken without food, hypoglycaemia (low blood glucose levels) will occur [ 76 ], but if food is taken without insulin, hyperglycaemia (high blood glucose levels) will occur [ 77 ]. A consistent, balanced diet that includes fibre and protein is important for maintaining glycaemic control.
“See , sometimes we would eat two times and other times have only porridge for the day [lunch or supper] , and I do not know how to match her insulin to that … . That has been a very big puzzle” - Caregiver [ 68 ]
Insulin dosing that is not aligned with carbohydrate intake can be fatal. Even in HICs where PWT1D have uninterrupted access to food, severe hypoglycaemia accounts for up to 10% of deaths among youths [ 78 ]. The fear of hypoglycaemia and hyperglycaemia mean that at times, PWT1D in humanitarian settings have the impossible choice of choosing between starvation or eating the “wrong” foods.
“But for her , she’s from a poor family . Her family cannot afford all those vegetables , those extra things to balance her diet . So sometimes she keeps herself hungry because she doesn’t want to eat rice for her sugar to raise . ” –Interpreter for PWT1D [ 59 ]
This means that even when insulin is available, affordable, or provided without cost to patients, and stored correctly, food insecurity can render insulin therapy ineffective, or worse, life-threatening.
Theme 2: Family Caregiving
Financial pressure..
T1D’s substantial financial burden is felt by all members of the family, often forcing parents to make unthinkable decisions about which children to support. In many cases, access to food, insulin, and medical supplies–the prerequisites of T1D management–is contingent on the ability to pay.
One father explained that he had to neglect his child’s T1D care to provide for the rest of his family.
“We have no money to get treatment for our daughter . I don’t have any work . We are advised to go to the hospital twice a year but this year we didn’t go . The last time we were at the hospital two years ago , it did cost 2000 Somoni (430 USD) . Father of a child with T1D [ 64 ]
Where parents were able to pay for diabetes management supplies, it often required finding additional income streams.
“My husband has two jobs: at night he works in the municipality and during the day he works in the ice cream factory . Sometimes we don’t have money , so we have to borrow money from relatives” . - Mother of a child with T1D [ 62 ]
Or taking financial support away from other children.
“Each month , 600 shekels is deducted for my son’s treatment , although I have a big family . I always wonder how I will meet their needs . If my son did not have diabetes , I could use this money for my daughter , who is going to university” . Father of a child with T1D [ 62 ]
Many families needed to make appalling choices under the principle of opportunity cost–the choice between diabetes care for one child or meeting the basic needs of the family is a deplorable scenario that is all too common [ 79 ].
Gender norms.
Gender expectations influenced parents’ expectations for their daughters’ futures, leading to differential treatment between sons and daughters diagnosed with T1D. Gender norms were a significant upstream barrier to T1D management and could not be overcome with individual-level facilitators like discipline.
For daughters, parents expressed anxiety about marriage prospects, citing that T1D compromises their daughter’s perceived suitability as wives and mothers.
“Her father was so sad , he felt that she will be a burden to him; how will she get married? I don’t think she will because she has diabetes . ” Mother of a child with T1D [ 62 ]
In some humanitarian settings, gendered cultural expectations made it difficult for female adolescents, but not males, to be physically active. Restricting daughters from leaving home and preventing them from participating in physical activity was common.
“I like walking , but my mother can’t so I can’t because girls can’t walk alone without a guardian . Also , she prevented me from going out walking with my friends . ” PWT1D [ 62 ]
In some communities, public physical activity is unacceptable for women [ 62 ]. Women with T1D who break social conventions to try and improve their glycaemic control could face significant familial and social implications.
Mental health and psychosocial burden- including for caregivers
Many family members discussed the overwhelming difficulty of caring for loved ones with T1D.
“[My wife] gets discouraged . She wonders for how long this will continue . She figures I am already gone , living on medicines all my life . It brings her worry … The diet brings a lot of strife . Because she has to prepare her food with the children , then prepare mine . It brings many quarrels . ” PWT1D [ 66 ]
Some parents, particularly mothers, spoke about how their child’s diagnosis with T1D led them to reduce social contact with their communities. The unequal social and emotional burden on mothers further highlights the gendered differences in T1D management. Because community events were often centred around “unhealthy” food, it was difficult for children with T1D to stick to their approved diet. Thus, parents chose to sacrifice their attendance at social events to promote their child’s diet adherence and protect their families from judgement.
“For today , and tomorrow I will not go to social events such as weddings . Even my neighbours are disappointed with me because I don’t visit them . Even my mother , I don’t visit her , especially when my son doesn’t commit to the treatment . ” - Mother of a child with T1D [ 62 ]
Social and community support for families managing T1D was often poor due to insufficient knowledge and T1D stereotypes, leading to feelings of depression, anxiety, and isolation among PWT1D and their families.
Many parents also noted significant distress from seeing their children receive insulin injections. Although many parents knew about the necessity of insulin for T1D management, seeing their child in pain led to missed insulin doses.
“My daughter cried when I gave her injections . She cried “Don’t do that! Don’t do that!” Because my heart burned , I could not do the insulin injections . ” Mother of a child with T1D [ 64 ] “For almost one month he did not get insulin injections, because we just didn’t want to hurt him anymore, but then he got very, very sick.” Father of a child with T1D [ 64 ]
Few family members were aware of T1D complications and alleviating the immediate felt pain of injections was therefore prioritized over preventing long-term complications.
Theme3: Knowledge Translation
T1d knowledge by pwt1d..
A lack of knowledge translation, known as the dynamic process, that includes the synthesis, exchange, and application of knowledge to improve health, was a recurring theme across all eleven studies. Minimal knowledge about diabetes complications wasn’t an experience unique to family members–many PWT1D were never provided with an opportunity to learn about what T1D was and how mismanagement could lead to complications.
“[My diabetes] wasn’t stable when I was young . I told you , I didn’t know about eating rice and white bread . All of this increased diabetes levels . I didn’t measure my glucose levels all the time . I did not know what it was , what it meant , and its complications . I used to know that I had to take the medication , eat normally and cut out sweets only . I did not know that carbohydrates increased the glucose level . ” –PWT1D [ 63 ]T1D Knowledge by Healthcare Providers
Low T1D knowledge at the patient level was indicative of a knowledge translation gap between healthcare providers and PWT1D, but also of a system-wide insufficiency in T1D knowledge.
One father described his despair when his daughter with undiagnosed T1D was transferred from facility to facility, receiving treatments for a variety of conditions–malaria, measles, intestinal worms, allergies, and typhoid fever–until finally falling into a coma due to healthcare provider-induced severe hyperglycaemia.
“Wherever we had been , the hospital , the diagnostic centre and other places , nobody found out what was going on […] When we went to this other hospital , they said they needed to “wash and clean” her stomach and she got a glucose infusion . After that she went into a coma . ” Father of a child with T1D [ 64 ]
The lack of knowledge about T1D and delayed diagnoses meant that many PWT1D in humanitarian settings experienced complications requiring serious clinical intervention. Because health services were often run by ad hoc health centres and short-term humanitarian aid clinics, PWT1D experienced little standardization in treatment:
“Specialists have studied in different hospitals around the world , and each doctor follows the methodology taught at that hospital , resulting in a variety of procedures and prescriptions . Only a few doctors prefer to have standardized procedures . ” –Ministry of Health Official [ 60 ]
The combination of siloed healthcare providers and under-resourced health services also invited a lack of accountability, leading to medical “shortcuts”.
“Too many cases of diabetic foot amputation in Syria… The problem is that surgeons taking care of the diabetic foot have poor skills in treating infections . Therefore , they go with the easier solution , which is amputation instead of treatment , especially as there is no accountability . ” –Ministry of Health Official [ 60 ]
In high-income settings where health services are comparatively more accessible than in many humanitarian settings, people with diabetes who undergo a major amputation tend to have a 5-year survival rate of less than 50% [ 80 ].
T1D Knowledge by Policymakers
The lack of health providers trained about T1D reflects a long-standing prioritization of infectious diseases such as HIV, malaria, and tuberculosis. Due to under-funded health systems and resource allocation towards communication diseases, there is a paucity of data demonstrating the T1D burden in many humanitarian settings.
“Often people will die very young without being diagnosed . As a result , people don’t see those cases , and when you want to do advocacy they will tell you we don’t see those cases . But we know those cases occur , but before they are diagnosed they pass off and they are buried and then no one knows them… The figures are not there , the people don’t see it , so it’s difficult to raise awareness , and because we are not responding , people are not aware , so it’s a kind of circle…” Policymaker, [ 59 ]
In LMICs, T1D registries are rare. When data on diabetes is collected, T1D and T2D are generally grouped together, further hiding the true prevalence of T1D [ 59 ].
Theme 4: Response to Diagnosis
T1D is not just hidden at the health-system data collection level. It is also commonly hidden on an intrapersonal level. Many of the PWT1D in the studies identified concealed their diagnosis from their social and familial network due to fears of rejection and stigmatization. Although there was sometimes pressure to disclose their diagnosis, PWT1D went out of their way to keep their diagnosis hidden.
Concealing diagnosis.
“One of my classmates told me that I’m a diabetic but I denied it . … I’m afraid that if my friends’ parents know that I have diabetes , they might prevent their children from talking to me , and say I am sick” . PWT1D [ 62 ]
School-aged youths with T1D identified alienation from friends and classmates as a central contributor to their psychosocial stress [ 59 ]. They reported anxiety about their inability to eat the same foods and do the same activities as their peers. Indeed, when T1D diagnoses were revealed at school, children faced consequences.
“The teacher stopped me from playing football because he is afraid I will have hypoglycemia or become dizzy… Sometimes I cry , because I love sport . ” PWT1D [ 62 ]
Children both anticipated and experienced stigma due to their diagnoses. In some cases, youths with T1D isolated themselves from their friends for self-protection.
“My life changed because since I was diagnosed in 2014 , I stopped going out with my friends… its always ‘My man , that stuff I can’t eat , I don’t do that . ’ Someone else , ‘Oh my man you’re selfish . ’ But I really didn’t want to tell him why the reason I was doing it… And it hurt me a lot . ” PWT1D [ 59 ]
Fears of stigma and discrimination about T1D were so strong that some asserted that they would not tell others about their diagnosis, even in the case of a hypothetical disaster.
“I do not want to say that I am diabetic when a disaster strikes . ” PWT1D [ 67 ]
Like the psychosocial burden felt by families of children with T1D, poor support in social contexts led to poor mental health outcomes for PWT1D.
Emotional response.
Dealing with shame, stigma, and exclusion are aspects of diabetes distress (DD), a condition used to refer to the accumulation of daily stresses that individuals with diabetes experience while managing their diabetes [ 81 ]. DD captures the feelings of powerlessness, overwhelm, frustration, and anger that result from living with T1D [ 82 ]. Though there are no published meta-analyses about the prevalence of DD among PWT1D, it is estimated that between 20 and 40% of the T1D population experience elevated DD [ 81 ].
“My daughter is often very sad about her situation . Sometimes she says that she would rather die . ” PWT1D [ 64 ] “Sometimes she feels so bad. You know, when she’s at play and I call her to come and do the test, she says, ‘Hey man, I’m tired with this sickness, I just want to die now. Every day, all my fingers hurting, I’m tired.” Caregiver [ 59 ]
Although DD varies across populations of PWT1D, being female, being young, and having weak social or familial support systems (e.g. living with an unsupportive partner, having an uninformed family, perceiving a lack of help in one’s social support network) are significantly associated with higher levels of DD [ 83 , 84 ]. These characteristics were common across the PWT1D interviewed in the included studies.
Recommendations
This synthesis of lived experiences of PWT1D and their caregivers, and the tacit knowledge of health stakeholders in humanitarian settings reveals several barriers to coping and care: access to insulin and food, familial and cultural constraints, information gaps, clinical management, and illness coping. We discuss how these findings can be translated into policies and programs that address the unmet need for material, psychological and health support among PWT1D in humanitarian settings.
Remove the need for ‘trade-offs’ by addressing economic costs, and acting on the multi-level commercial determinants of food and insulin insecurity
Glycaemic control is often discussed in relation to “medication adherence”, defined as the extent to which patients follow prescribed medication dosing regimens [ 85 ]. As was highlighted in interview excerpts, adhering to T1D management routines in many humanitarian settings was often effectively unattainable due to food insecurity, insulin and daily glusose testing accessibility, among other challenges. Rationing insulin was a common coping mechanism in the face of medication and food insecurity, a finding that is aligned with existing literature on T1D management strategies in under-resourced settings [ 52 ]. When insulin was not available, or food options were not conducive to glycaemic control, PWT1D sometimes opted to skip meals entirely.
Adherence, whether to healthy eating or to insulin regimes, is a privilege, and for many in low resource and humanitarian settings it is not a choice or agentic decision, but a trade-off [ 86 ]. Non-adherence narratives can inadvertently allocate blame to the patients, taking attention away from the significant, contextual barriers to diabetes management. Researchers, healthcare providers, and policymakers must understand the economic context in which these trade-offs are made.
Existing guidelines for NCD management in humanitarian settings can be updated to include these nuanced considerations of T1D experience and program needs. An immediate way to remove some constraints can be through providing customised packages of self-care and nutritional resources (e.g., vouchers or provisions to access transport and food banks or regular provision of nutritious meals, infrastructure to store insulin, supplies of test strips and needles). These provisions are necessary to address the economic component of T1D management outside of health facilities–that is, where people live and work. The bigger and more time-consuming task lies at the national structural and international trade policy level–that is to enforce policies and programs that directly target the factors blocking sustainable and affordable access to insulin and healthy food–the commercial determinants of insulin and healthy food.
Economic constraints, and catastrophic out-of-pocket health payments is not unique to T1D management, and is also experienced by people living with T1D outside of humanitarian settings to various degrees [ 53 ]. These overarching discussions relate to the management of T2D and many other NCDs, mental health and neurological conditions. There is ongoing policy calls and advocacy, such as through past and upcoming dialogues on ‘Sustainable Financing for NCDs and Mental Health’. Chronic conditions can impose significant economic development and productivity burden, and more effective financing strategies are needed to address the NCD investment gaps globally [ 87 ].
Provide family and community-based mental health support and awareness tools for individual self-care, family-based caregiving, and managing stigma and psychosocial distress
This theme runs parallel with the expanding literature base that characterizes T1D as a “family affair” due to the impact of its management regime on all members of the household [ 88 , 89 ]. A key point of difference between T1D and T2D is the life course stage at which the disease is often diagnosed or managed at–childhood, adolescence, and young adulthood. Involving and engaging caregivers in the diagnosis and management processes is imperative to achieve positive illness coping. The family is the main source of medical, emotional, and financial support for PWT1D –particularly when health and social welfare systems fail at providing necessary care. Knowledge of family dynamics is critical when considering approaches to promoting T1D care in humanitarian settings. Interventions that exclusively target PWT1D, rather than PWT1D with their family and caretakers, can fall short by failing to address management barriers related to familial or cultural norms. Healthcare providers and community health workers or volunteers need to be aware of the stigma and cultural contexts that shape family involvement in T1D management.
PWT1D, caregivers, and healthcare providers discussed distress related to the T1D diagnosis and subsequent management. This theme echoes findings from previous studies which have shown that PWT1D and their caregivers sometimes actively hide a T1D diagnosis for fear of being characterized as intolerable burdens (e.g., chronically sick, a reminder of death), inferior marriage material (e.g., high-risk pregnancies, unable to provide financially for the household), and depressing people to be around (e.g., unable to enjoy certain foods and activities) [ 62 , 90 ]. Stigma is also strongly related to psychological distress among people with all types of diabetes [ 91 ]. Even in stable settings, PWT1D have significantly higher rates of mental health conditions including depression, anxiety, and suicide compared to those without T1D [ 92 ]. Poor mental health is associated with poor diabetes management and fewer health-seeking behaviours, both of which are important in humanitarian settings where health support systems are diminished [ 93 ]. These findings reiterate literature emphasizing the importance of post-diagnosis treatment support for people with chronic diseases and their families in under-resourced settings [ 94 , 95 ].
Future programs and policies should not just be patient-centred , but also, family-centred . A strong focus on families, their education and psychosocial support in managing T1D as a family and household unit is required to promote sustainable T1D management. Healthcare providers should endeavour to create personalized T1D treatment plans that intimately considers the familial and cultural norms, while also investing in community and structural level awareness programs that can alleviate the social stigma surrounding PWT1D.
For this, culturally congruent, innovative, and frugal programs that facilitate the delivery of diabetes education and psychosocial support should be considered, especially where on-site health staff capacity is limited. These include peer support groups, e-health programs, and talk-therapy delivered by trained lay health workers or community members. Program and policies must be developed with the goal of fostering community awareness and education on T1D, dispelling myths related to the condition. Visible involvement and mass communications by trusted and respected community or religious leaders, and other positive social influencers can be leveraged to disseminate accurate information and encourage healthy and care-seeking behaviours. Peer support programs that integrate approaches for daily management and social and emotional support have been used to promote the management and coping of other chronic conditions [ 96 , 97 ]. Additionally, lay-delivered talk therapies for PWT1D and their families can also be beneficial, with similar programs conducted in humanitarian settings being seen as feasible to implement, and acceptable by service users [ 98 ].
T1D is prevalent in girls and boys in near equal proportions, but gender norms may create inequalities in coping and management. Programs that include the promotion of gender equality and address restrictive gender norms that affect illness coping and management, including those involving men and boys, can also be beneficial for promoting individual and community-level T1D management [ 99 ]. Drawing lessons and evidence from the HIV sector, it is also important to act on structural, educational, and economic domains that can accumulatively contribute to positive health outcomes. This can include abolishing school fees, extending compulsory education, cash transfers, microfinance and income-generating activities such as skill-building and vocational training, programs to address gender-based violence, through community-based participatory learning, peer and partner discussions [ 100 , 101 ]. Programs must be context-relevant and acceptable among the community, and should include societal and cultural themes of experience such as anxieties related to marriage prospects and societal reputation. Importantly, these programs must be informed and designed based on the meaningful involvement of the people and families that live with T1D themselves—which is overall still a less common practice in humanitarian settings and many LMICs [ 102 ].
Invest in and provide T1D relevant capacity building, knowledge generation and translation to practice for healthcare workforce and policymakers
Few studies provided insights into the tacit knowledge of healthcare workers and policy makers, suggesting that working knowledge about T1D was not strong among healthcare providers and policymakers across the humanitarian settings. As literature from other low-income settings has highlighted, healthcare providers often lack opportunities to learn fundamental knowledge about T1D, leading to misdiagnoses, limited patient education, and poor patient T1D management skills [ 64 ].
In humanitarian settings, low professional knowledge and uncoordinated clinical practices are accompanied by limited data and disease surveillance. In 2018, the Humanitarian NCD Interagency Study in Emergencies and Disasters (UNITED) found that less than 50% of 83 surveyed humanitarian sites distinguished between T1D and T2D, and less than 30% reported on GV complications [ 103 ], further supporting the necessity of strengthened T1D knowledge translation in humanitarian settings.
To address these gaps, efforts to sensitize healthcare providers at primary, community, and tertiary health care levels, and strengthen knowledge translation at provider and political levels, is necessary. This can include capacity-building and training initiatives, and accessible educational materials (e.g., infographics) about T1D stereotypes, insulin dosing, and complications. Creating and disseminating T1D management curriculums for healthcare providers (e.g., nurses, doctors, and community health workers) and establishing guidelines to standardize systems of care is necessary to improve the coordination and accountability of T1D care in humanitarian settings. The NCDI (non-communicable disease and injuries) Poverty Network and the PEN-PLUS partnership is an example of a global initiative that is mobilising resources, building awareness, and developing local health workforce capacity to reduce the burden of complex chronic conditions like T1D [ 104 – 106 ].
Limitations
This qualitative synthesis has taken a transparent, systematic approach to synthesizing qualitative evidence about the experiences of PWT1D in humanitarian settings; filling a significant gap in the research base. Nevertheless, we acknowledge several limitations in our approach.
Despite a comprehensive search of five databases, relevant studies may have been missed. First, our definition of humanitarian setting and subsequent database search strategy may have missed relevant studies. This is not an exhaustive systematic review, but rather a qualitative synthesis of papers that richly portray the nuanced lived experiences of PWT1D, their caregivers, healthcare providers, and policymakers in humanitarian settings. Diabetes is also a heterogeneous disease with large variations in beta cell dysfunction and insulin resistance, which can lead to conflation between T1D and T2D diagnoses [ 107 ]. In humanitarian settings, sites also often only collect aggregate data on the number of diabetes consultations, rather than the number of patients with T1D or T2D, which can lead to the generalization of all diabetes to T2D [ 103 ]. Both circumstances can lead to the misclassification of T1D as T2D, meaning that articles excluded due to a focus on T2D would have been missed in the research process.
Further, grey literature and unpublished reports were not included in this meta-synthesis, which may have further provided insight into the lived experiences of PWT1D in humanitarian settings. Limiting this exercise to studies published in English also means that key insights provided in other languages were not included. A future review could expand upon this work by searching the grey literature and by including non-English publications.
Despite these limitations, this article provides a rich synthesis exploring the contextual, cultural, gendered, and health system factors that impact T1D management in humanitarian settings. Future research on how to address barriers to T1D management in humanitarian settings would be enhanced by more comprehensive focus on the experiences of the humanitarian organizations, donors, public and private health actors, and local NGOs who work in the field.
This paper consolidates narratives of lived experience and tacit knowledge from PWT1D, their caregivers, healthcare providers, and policy makers, illuminating the mounting health needs of people impacted by humanitarian crises. The narratives and themes in this paper demonstrate how T1D management intimately reflects, and is contingent on the broader familial, cultural, and economic systems in which it exists. Policymakers and healthcare providers should be aware of the severe trade-offs that PWT1D and their families make in the name of diabetes management. In particular, NCD programs that strive to manage T1D must also include arrangements fro sustainable and continuous access to crucial needs such as insulin, glucose testing kits and strips, as well as nutritional goods or food vouchers. Additionally, effective management strategies in a younger (sometimes pediatric) population, with interventions that engage and involve their parents as caregivers, primary health care service providers, and broader members of society including their school peers, teachers, and neighbours is necessary.
The recommendations and insights outlined in this report are also relevant to the management of other NCDs, including T2D in many resource-constrained settings. Existing guidelines and frameworks for action can be updated to include notes on the differences and similarities of needs in managing T1D, T2D, other NCDs, mental health and neurological conditions, particularly where negative outcomes are known to be amplified by experiences of deprivation and poverty. Targeted action through joint multi-sectoral investment can address some of the root constraints and barriers to care outlined in this paper. We sincerely hope that these insights will contribute to the development of stronger T1D aid and resources for PLWT1D, their families, their communities, and health stakeholders in all forms of humanitarian settings.
Supporting information
S1 text. literature search strategy for medline (ovid) database..
https://doi.org/10.1371/journal.pgph.0003027.s001
S2 Text. Images of manual analysis process-establishing codes, sub-themes, and themes.
https://doi.org/10.1371/journal.pgph.0003027.s002
- View Article
- Google Scholar
- 3. Inter-agency Working Group. Inter-Agency Field Manual on Reproductive Health in Humanitarian Settings: 2010 Revision for Field Review. Geneva: Inter-agency Working Group on Reproductive Health in Crises; 2010. Available: http://www.ncbi.nlm.nih.gov/books/NBK305149/
- PubMed/NCBI
- 37. Kleinman A. The Illness Narratives: Suffering, Healing, And The Human Condition. Reprint edition. New York: Basic Books; 1989.
- 38. Kosciulek JF. The social context of coping. Coping with Chronic Illness and Disability. Springer; 2007. pp. 73–88. Available: http://link.springer.com/content/pdf/10.1007/978-0-387-48670-3_4.pdf
- 45. Jutel AG, Conrad P. Putting a Name to It: Diagnosis in Contemporary Society. 1 edition. Baltimore: Johns Hopkins University Press; 2011.
- 46. Manderson PL, Smith-Morris PC. Chronic Conditions, Fluid States: Chronicity and the Anthropology of Illness. New Brunswick: Rutgers University Press; 2010.
- 50. World Health Organization. Everybody’s business—strengthening health systems to improve health outcomes: WHO’s framework for action. World Health Organization; 2007. Available: https://apps.who.int/iris/handle/10665/43918
- 51. World Health Organization. Monitoring the building blocks of health systems: a handbook of indicators and their measurement strategies. Geneva: World Health Organization; 2010. Available: https://apps.who.int/iris/handle/10665/258734
- 96. Evans M, Daaleman T, Fisher EB. Peer Support for Chronic Medical Conditions. In: Avery JD, editor. Peer Support in Medicine: A Quick Guide. Cham: Springer International Publishing; 2021. pp. 49–69. https://doi.org/10.1007/978-3-030-58660-7_3
This paper is in the following e-collection/theme issue:
Published on 9.7.2024 in Vol 26 (2024)
Telemedicine in Improving Glycemic Control Among Children and Adolescents With Type 1 Diabetes Mellitus: Systematic Review and Meta-Analysis
Authors of this article:

- Kun Zhang 1 , MSN ;
- Qiyuan Huang 1 , MD ;
- Qiaosong Wang 1, 2 , MSN ;
- Chengyang Li 1 , MSN ;
- Qirong Zheng 1 , MSN ;
- Zhuoyue Li 3 , MM ;
- Dan Xu 4 , MD ;
- Cuiling Xie 1 , MSN ;
- Mingqi Zhang 1 , MSN ;
- Rongjin Lin 1, 2 , BM
1 School of Nursing, Fujian Medical University, Fuzhou, China
2 Department of Nursing, The First Affiliated Hospital of Fujian Medical University, Fujian Medical University, Fuzhou, China
3 Department of Infectious diseases, Nanfang Hospital, Southern Medical University, Guangzhou, China
4 Foreign Language Department, Xuzhou Medical University, Xuzhou, China
Corresponding Author:
Rongjin Lin, BM
School of Nursing
Fujian Medical University
No.1 Xuefu North Road, Minhou County
Fuzhou, 350005
Phone: 86 1 380 950 8580
Fax:86 0 591 228 62526
Email: [email protected]
Background: Type 1 diabetes mellitus (T1DM) is the most common chronic autoimmune disease among children and adolescents. Telemedicine has been widely used in the field of chronic disease management and can benefit patients with T1DM. However, existing studies lack high-level evidence related to the effectiveness of telemedicine for glycemic control in children and adolescents with T1DM.
Objective: This study aims to systematically review the evidence on the effectiveness of telemedicine interventions compared with usual care on glycemic control among children and adolescents with T1DM.
Methods: In this systematic review and meta-analysis, we searched PubMed, Cochrane Library, Embase, Web of Science (all databases), and CINAHL Complete from database inception to May 2023. We included randomized controlled trials (RCTs) that evaluated the effectiveness of a telemedicine intervention on glycemic control in children and adolescents with T1DM. In total, 2 independent reviewers performed the study selection and data extraction. Study quality was assessed using the Cochrane Risk of Bias 2 tool. Our primary outcome was glycated hemoglobin (HbA 1c ) levels. Secondary outcomes were quality of life, self-monitoring of blood glucose, the incidence of hypoglycemia, and cost-effectiveness. A random-effects model was used for this meta-analysis.
Results: Overall, 20 RCTs (1704 participants from 12 countries) were included in the meta-analysis. Only 5% (1/20) of the studies were at high risk of bias. Compared to usual care, telemedicine was found to reduce HbA 1c levels by 0.22 (95% CI –0.33 to –0.10; P <.001; I 2 =35%). There was an improvement in self-monitoring of blood glucose (mean difference [MD] 0.54, 95% CI –0.72 to 1.80; P =.40; I 2 =67.8%) and the incidence of hypoglycemia (MD –0.15, 95% CI –0.57 to 0.27; P =.49; I 2 =70.7%), although this was not statistically significant. Moreover, telemedicine had no convincing effect on the Diabetes Quality of Life for Youth score (impact of diabetes: P =.59; worries about diabetes: P =.71; satisfaction with diabetes: P =.68), but there was a statistically significant improvement in non–youth-specific quality of life (MD –0.24, 95% CI –0.45 to –0.02; P =.04; I 2 =0%). Subgroup analyses revealed that the effect of telemedicine on HbA 1c levels appeared to be greater in studies involving children (MD –0.41, 95% CI –0.62 to –0.20; P <.001), studies that lasted <6 months (MD –0.32, 95% CI –0.48 to –0.17; P <.001), studies where providers used smartphone apps to communicate with patients (MD –0.37, 95% CI –0.53 to –0.21; P <.001), and studies with medication dose adjustment (MD –0.25, 95% CI –0.37 to –0.12; P <.001).
Conclusions: Telemedicine can reduce HbA 1c levels and improve quality of life in children and adolescents with T1DM. Telemedicine should be regarded as a useful supplement to usual care to control HbA 1c levels and a potentially cost-effective mode. Meanwhile, researchers should develop higher-quality RCTs using large samples that focus on hard clinical outcomes, cost-effectiveness, and quality of life.
Introduction
Type 1 diabetes mellitus (T1DM) is the most common chronic autoimmune disease among children and adolescents, characterized by hyperglycemia and caused by an absolute deficiency of insulin [ 1 , 2 ]. More than 1.2 million children and adolescents worldwide currently have T1DM [ 3 ]. Adolescence is a period when glycemic control commonly deteriorates [ 4 ], and people with diabetes remain at high risk of serious complications, including diabetic cardiovascular disease and diabetic nephropathy [ 5 - 7 ]. T1DM has a serious impact on the life health of children and adolescents. It places a heavy medical burden on the families of those affected [ 8 , 9 ]. Therefore, there is an imperative to explore effective treatment together with management strategies to help children and adolescents maintain normoglycemia and promote their long-term health as well as their well-being.
In recent years, telemedicine has been widely used in the field of chronic disease management. Telemedicine (a subcomponent of eHealth) has been defined as “The delivery of health care services, where distance is a critical factor, by all health care professionals using information and communications technologies for the exchange of valid information for diagnosis, treatment and prevention of disease and injuries, research and evaluation, and the continuing education of health care workers, with the aim of advancing the health of individuals and communities” [ 10 ]. For patients with chronic diseases, the advantages of telemedicine can be reflected in improving access to services, ensuring continuity of care, and mitigating the costs of care delivery [ 11 , 12 ]. Although telemedicine may not be able to provide physicians with comprehensive diagnostic information about a patient in the same way that a physical examination can, it can assist physicians in monitoring and recording certain specific physiological indicators (eg, blood glucose, blood oxygen concentration, blood pressure, and heart rate) to help them observe the trajectory of a patient’s health [ 13 , 14 ]. The current studies on telemedicine interventions for glycemic control in patients with diabetes focus on (1) telemonitoring (eg, a web-based telemedicine system was used to monitor patients with T1DM in the study by Ruiz de Adana et al [ 15 ]), (2) tele-education (eg, Molavynejad et al [ 16 ] delivered tele-education to patients with diabetes using remote video-based technology), and (3) teleconsultation and internet-based group appointments (eg, Bisno et al [ 17 ] provided both individual telehealth provider visits and internet-based group appointments for patients with T1DM through the CoYoT1 clinic). Moreover, previous meta-analyses have shown that the effectiveness of telemedicine in controlling blood glucose levels in patients with T1DM has been well validated [ 18 - 20 ]. It can be seen that telemedicine provides a huge advantage for diabetes glycemic control.
However, existing studies lack high-level evidence related to the effectiveness of telemedicine for glycemic control in children and adolescents with T1DM. Only a few studies have reported on the potential of telemedicine in the management of T1DM in children and adolescents. Moreover, the safety and applicability of telemedicine for children and adolescents with T1DM need to be further demonstrated. Therefore, we aimed to conduct a systematic review and meta-analysis of current randomized controlled trials (RCTs) to provide new evidence for clinical decision-making by comparing the effectiveness of telemedicine interventions with usual care in children and adolescents with T1DM.
Study Question
How does telemedicine compare with usual care in improving glycemic control among children and adolescents with T1DM? Which form of telemedicine intervention is more effective in improving glycemic control among children and adolescents with T1DM?
Study Objective
This meta-analysis aimed to comprehensively synthesize and evaluate evidence on the effectiveness of telemedicine on glycemic control among children and adolescents with T1DM.
Search Strategy
In total, 5 electronic databases covering the realms of biomedicine science, clinical medicine science, and general references were screened: PubMed, Cochrane Library, Embase, Web of Science (all databases), and CINAHL Complete. The dates searched were from establishment of each database to May 1, 2023. The search was conducted using the following keywords: (“Diabetes Mellitus, Type 1”) AND (“Telemedicine” OR “Telemetry” OR “Telenursing” OR “Internet-Based Intervention”) AND (“Child” OR “Adolescent”). Medical Subject Heading terms and their related terms were used. Multimedia Appendix 1 [ 21 - 40 ] shows the detailed search terms and search process. There were no restrictions in terms of participant age, year of publication, or region of study at this stage. The review protocol was reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 checklist ( Multimedia Appendix 2 ).
Inclusion and Exclusion Criteria
The inclusion criteria were defined by population, intervention, comparison, outcome, and study design as follows:
- Population: the target participants were children (aged ≤10 years) and adolescents (10 years<age≤19 years) [ 41 ] with T1DM.
- Intervention: complete or partial telemedicine intervention. A complete telemedicine intervention was one in which there was no face-to-face contact between the participants and the health care providers throughout the trial period from baseline to the end of the intervention and the only telemedicine interventions were via telephone, web-based videoconferencing, a website, or a smartphone app (all treatments [including initial treatment] were delivered via telemedicine). Partial telemedicine intervention referred to treatments that combine telemedicine with nontelemedicine interventions (such as a follow-up visit in an outpatient clinic or a visit at home). These 2 broad categories of telemedicine interventions were further subdivided by the number of intervention forms. “Single” refers to the inclusion of only 1 form of telemedicine intervention, whereas “mixed” refers to the inclusion of ≥2 forms of telemedicine intervention. Complete telemedicine interventions were categorized as single and mixed complete telemedicine interventions; partial telemedicine interventions were categorized as single and mixed partial telemedicine interventions.
- Comparison: containing a comparison group with usual care, including a nontelemedicine intervention and health guidance only before discharge treated as a blank control.
- Outcome: we included all studies that reported serum glycated hemoglobin (HbA 1c ) levels as either their primary or secondary outcomes.
- Study design: only RCTs (parallel or crossover) were included.
The exclusion criteria were (1) studies using nonexperimental and quasi-experimental designs; (2) abstracts, brief reports, conference proceedings, conference papers, posters, and letters to editors; (3) studies on patients with gestational diabetes; and (4) studies published in languages other than English because of our lack of high-quality translational resources.
Study Screening
Throughout the screening processes, all studies included in the analysis were independently reviewed by 2 researchers (KZ and CL). First, we screened the titles and abstracts of all bibliographic records against the inclusion and exclusion criteria, and a label was created on a serial numbered sheet to add the reason for exclusion as a note. Second, we thoroughly read the full text of the study without exclusion labels to ensure that all inclusion and exclusion criteria were met. Disagreements between the researchers were resolved by meeting with a third reviewer (QH). Studies judged to be eligible at this stage were then included in the quality assessment where applicable.
Quality Assessment
We assessed the risk of bias using the Cochrane Risk of Bias 2 tool [ 42 ] to evaluate the randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results. In total, 2 researchers (KZ and CL) assessed the trials independently and resolved any disagreements by meeting with a third reviewer (QH). The quality of evidence of each study was assessed by 2 reviewers (QH and QW) using the Grading of Recommendations, Assessment, Development, and Evaluations approach [ 43 ].
The primary outcome was HbA 1c levels. Secondary outcomes were quality of life as measured using a validated instrument, daily frequency of self-monitoring of blood glucose (SMBG), the incidence of hypoglycemia, and cost-effectiveness.
Data Extraction
For each included study, 2 reviewers (KZ and CL) independently extracted the data for analysis. When data were missing or unclear, we contacted the authors. If the authors did not respond, the study was reassessed and excluded.
We extracted the following information from the selected studies: (1) study characteristics (study name, author, year of publication, country, study design, attrition rate, and sample size), (2) characteristics of the participants (age, gender, diabetes duration, baseline HbA 1c levels, total cholesterol levels, triglyceride levels, blood pressure, and BMI), (3) intervention details (duration, types of health care providers, frequency of feedback, characteristics of intervention content, communication forms between providers and patients, technology use modes, and telemedicine intervention forms; communication forms included modem, SMS text messaging, email, web conference, website—websites where patients upload blood glucose levels or other clinical data and share them with their health care providers—computer software, smart wearable devices—smart wearable devices are consumer-grade connected electronic devices that can be worn on the body as an accessory or embedded into clothing [ 44 ] —telephone, and smartphone or its apps), and (4) general information about outcomes (the mean and SD at baseline and at the end of the intervention, number of participants analyzed at the end of the intervention, and tools used for measurement; when several analyses were performed on the same outcome at the same time point, we extracted the data from the intention-to-treat analysis).
Data Analysis
Stata (version 17; StataCorp) and Review Manager (version 5.4; The Cochrane Collaboration) were used for all statistical analysis. For quantitative synthesis, we collected the difference between baseline and end-point values for both the intervention and control groups. In the absence of information, data were estimated from the mean and SD of baseline and end-point values using a correlation of 0.5 [ 45 ]. To ensure accuracy, different correlations, such as 0.4 and 0.6, were used for estimation data and sensitivity analysis. The final results showed that the estimated results obtained using the different correlations remained stable after sensitivity analysis [ 45 ]. Data conversion tools were used to convert the median, maximum, and minimum values reported in the included studies into mean [ 46 ] and SD [ 47 ]. We reported the results of secondary outcomes when data from at least 2 studies could be merged. The magnitude of the overall effect size was calculated based on the pooled mean difference (MD) with 95% CI when the same measures were used in the studies. If outcomes were measured using different outcome measurement scales, the pooled standardized MD (SMD) with 95% CI was adopted. A P value of <.05 was considered statistically significant.
A random-effects or fixed-effects meta-analysis for continuous data was performed based on the results of the heterogeneity test. Study heterogeneity was determined using the Cochran Q test and Higgins I 2 test. I 2 values of 25%, 50%, and 75% indicated low, moderate, and high heterogeneity, respectively [ 45 ]. If P >.10 and I 2 <50% were identified, fixed-effects models were used; otherwise, random-effects models were applied. To ensure the robustness of our results, a sensitivity analysis was performed by using leave-one-out analysis to assess the contribution of each study to the merged effect size.
Publication bias was assessed creating funnel plots, the Begg test, and performing the Egger regression test (considered significant at P< .05) by 2 reviewers (QW and CL), and agreement was reached through consensus [ 48 , 49 ]. For the primary outcome, we performed a series of subgroup analyses to quantify specific differences in the size of effects of particular telemedicine interventions based on study and intervention characteristics [ 50 ]. Moreover, we performed a univariable meta-regression analysis to investigate whether there was heterogeneity due to differences in study or intervention characteristics.
Protocol Deviation
First, the definition of the intervention group in the registration program as an Internet-based telemedicine intervention group is too broad and simplistic. After further research, we decided to categorize the interventions into complete and partial telemedicine interventions. These 2 broad categories of telemedicine interventions were further subdivided by the number of intervention forms. “Single” refers to the inclusion of only 1 form of telemedicine intervention, whereas “mixed” refers to the inclusion of ≥2 forms of telemedicine intervention. Complete telemedicine interventions were categorized as single and mixed complete telemedicine interventions; partial telemedicine interventions were categorized as single and mixed partial telemedicine interventions. These changes and clarifications help explain the impact of the “face-to-face contact between patient and healthcare provider” factor on telemedicine effectiveness during telemedicine interventions, which has important implications for the development of future telemedicine interventions.
Second, the definition of the control group (“usual care”) was also an oversimplification, so we illustrated 2 cases of “usual care” in this study, including a nontelemedicine intervention as well as health guidance only before discharge treated as a blank control.
Third, regarding secondary outcomes, initially, we identified secondary outcomes based on studies related to diabetes telemedicine in adults and other types of diabetes. However, during the literature reading, it was found that no studies analyzed blood pressure, weight, and patient satisfaction as study outcomes in telemedicine interventions on children and adolescents with T1DM. Some studies used only weight and blood pressure as baseline indicators and lacked postintervention data. Other studies only asked participants how satisfied they were with the telemedicine intervention through interviews at the end of the intervention, which prevented us from quantitatively assessing satisfaction. Therefore, secondary outcomes such as blood pressure, weight, and patient satisfaction were removed.
Finally, regarding the data synthesis strategy, we modified the section for missing data estimation. Because data for the primary and secondary outcomes were partially missing, we first used the commonly used correlation coefficient of 0.5 for data estimation according to the Cochrane Handbook for Systematic Reviews of Interventions [ 45 ]. However, as there is currently no clear specification for the use of correlation coefficients for data estimation (only a broad range of choices), to ensure that the effect sizes synthesized using the “estimated data” were sufficiently stable, we also used 0.4 and 0.6 as correlation coefficients for data estimation. (Our main purpose was to see whether the estimated effects using the new correlation coefficients would pass the sensitivity analyses after changing the correlation coefficient). The sensitivity analyses showed that the results synthesized after estimating the missing data using all 3 correlation coefficients were stable and reliable, but the data estimated using the more common correlation coefficient of 0.5 was used as the results of this study.
Search Results
The phases of electronic search, identification, and screening for eligible studies are depicted in the PRISMA flowchart ( Figure 1 ). A total of 546 studies were identified using the search strategy described previously. After removing duplicates and screening titles and abstracts, a total of 20 studies were retained for full-text evaluation. Finally, a total of 20 studies with 1704 participants were included in this systematic review and meta-analysis.

Study Characteristics
The characteristics of the studies are summarized in Table S1 in Multimedia Appendix 1 . A total of 90% (18/20) of the studies were parallel-group RCTs [ 21 - 38 ], and 10% (2/20) were crossover studies [ 39 , 40 ]. Of the 20 included studies, 12 (60%) were published after 2015. In total, 40% (8/20) of the studies were published in North America [ 21 - 24 , 27 , 29 , 30 , 34 ], 35% (7/20) were published in Europe [ 25 , 26 , 33 , 35 - 37 , 39 ], 20% (4/20) were published in Asia [ 28 , 32 , 38 , 40 ], and 5% (1/20) were published in Oceania [ 31 ]. The sample sizes of the studies ranged from 20 to 240, with the intervention periods ranging from 3 to 60 months. All participants included in the studies were aged <20 years and had T1DM. The median mean age at baseline was 13.5 years, and the median mean diabetes duration at baseline was 6.2 years. A total of 90% (18/20) of the studies were performed in adolescents (mean age 13.6; range 10.8-17.3 years), and 10% (2/20) of the studies were performed in children (mean age 5.8; range 5.6-6.1 years). The proportion of female participants at baseline ranged from 42% to 62%. The floored threshold value of baseline HbA 1c levels in 35% (7/20) of the studies was ≥7.5%.
Intervention Characteristics
The telemedicine systems used in most studies were relatively simple to operate, having clear processes and including transmission of blood glucose data with feedback (15/20, 75%) [ 21 - 24 , 26 , 28 , 29 , 31 , 33 - 37 , 39 , 40 ] or blood glucose data only (5/20, 25%) [ 25 , 27 , 30 , 33 , 38 ]. A specialist diabetes care team, including a diabetologist, nurse, dietician, and psychologist, was reported in 45% (9/20) of the studies [ 22 , 24 , 29 , 31 , 33 - 35 , 37 , 40 ]. Feedback was provided monthly or less frequently in 50% (10/20) of the studies [ 23 , 26 , 31 , 33 - 37 , 39 , 40 ] and every 2 weeks or more frequently in 25% (5/20) of the studies [ 21 , 22 , 24 , 28 , 29 ], and the frequency of feedback was not specified in 25% (5/20) of the studies ( Table 1 ) [ 25 , 27 , 30 , 32 , 38 ].
| Study, year, and country or region | Health care provider | Communication form | Frequency of feedback | Intervention content | Technology use mode | Technology form | Telemedicine intervention form | ||||||||||
| Provider to patient | Patient to provider | Internet-based follow-up | Medication adjustment | Diet guidance | Physical exercise | Basic health education | |||||||||||
| Chase et al [ ], 2003, United States | Nurse and physician | Telephone | Modem | Every 2 weeks | Yes | No | No | No | Yes | — | Hardware | Complete telemedicine intervention (single) | |||||
| Gandrud et al [ ], 2018, United States | Diabetes educator, nurse, and physician | SMS text messaging and email | Smartphone app | Weekly | Yes | Yes | No | Yes | Yes | — | Software | Partial telemedicine intervention (mixed) | |||||
| Goyal et al [ ], 2017, Canada | Human factors specialist, nurse, and physician | Smartphone app and telephone | Smart wearable device | Every 3 months | Yes | No | No | No | No | Independently | Software | Partial telemedicine intervention (mixed) | |||||
| Han et al [ ], 2015, United States | Diabetes educator, nurse, and physician | SMS text messaging | Smartphone app and SMS text messaging | Every 2 days | Yes | Yes | No | No | Yes | Independently | Software | Complete telemedicine intervention (single) | |||||
| Ibrahim et al [ ], 2021, Europe | Diabetologist | SMS text messaging | — | — | No | No | No | No | No | Independently | Software | Partial telemedicine intervention (single) | |||||
| Klee et al [ ], 2018, Switzerland | Nurse and diabetologist | Smartphone app and website | Telephone and email | Monthly | Yes | Yes | Yes | No | No | — | Software | Partial telemedicine intervention (mixed) | |||||
| Kowalska et al [ ], 2017, Poland | Pediatrician and diabetologist | Computer software | Smart wearable device | Every 13 weeks | Yes | Yes | Yes | No | No | Parental assistance | Software | Partial telemedicine intervention (single) | |||||
| Kumar et al [ ], 2004, United States | Trained research assistant | Website | Modem and smart wearable device | — | No | Yes | Yes | No | No | Parental assistance | Software | Complete telemedicine intervention (single) | |||||
| Landau et al [ ], 2012, Israel | Dietitian and pediatric endocrinologist | Telephone | Website and smart wearable device | Every week | Yes | Yes | No | No | No | Independently | Software | Partial telemedicine intervention (mixed) | |||||
| Marrero et al [ ], 1995, United States | Pediatric diabetologist, nurse, social workers, and dietitians | Computer software and telephone | Modem | Every 2 weeks | Yes | Yes | Yes | No | No | — | Software | Partial telemedicine intervention (mixed) | |||||
| Mulvaney et al [ ], 2010, United States | Diabetes professionals | Website | Website | — | No | No | No | No | Yes | — | Software | Complete telemedicine intervention (single) | |||||
| Nunn et al [ ], 2006, Australia | Pediatric endocrinologists, nurse, dietitian, and social worker | Telephone | Telephone | Every 2 months | Yes | Yes | Yes | Yes | Yes | Parental assistance | Hardware | Complete telemedicine intervention (single) | |||||
| Raviteja et al [ ], 2019, India | Consultant and physician | Smart wearable device | Smart wearable device and computer software | — | Yes | Yes | Yes | Yes | No | Independently | Hardware | Complete telemedicine intervention (mixed) | |||||
| Schiaffini et al [ ], 2016, Italy | Diabetologist, nurse, dietician, and psychologist | Web conference | Website and smart wearable device | Every month | Yes | No | Yes | Yes | Yes | Parental assistance | Software | Complete telemedicine intervention (mixed) | |||||
| Shalitin et al [ ], 2014, Israel | Diabetes care team | Website, email, and telephone | Smart wearable device and website | Every month | Yes | Yes | No | No | No | Parental assistance | Software | Complete telemedicine intervention (mixed) | |||||
| Stanger et al [ ], 2018, United States | Pediatric endocrinologist and diabetes care team | Web conference | Smart wearable device | Every month (last period) | Yes | Yes | No | No | No | Parental assistance | Software | Complete telemedicine intervention (single) | |||||
| Von Sengbusch et al [ ], 2020, Germany | Regular home diabetes team | Web conference | Smart wearable device and computer software | Every month | Yes | Yes | No | Yes | Yes | Parental assistance | Software | Partial telemedicine intervention (single) | |||||
| Ware et al [ ], 2022, United Kingdom | Nurse and physician | Smartphone app, telephone, and email | Smartphone app and smart wearable device | Every month | Yes | Yes | No | No | No | Parental assistance | Software | Complete telemedicine intervention (mixed) | |||||
| Ware et al [ ], 2022, United Kingdom | Research team and clinical team | Smartphone app, telephone, and email | Smartphone app and smart wearable device | Every month | Yes | Yes | Yes | No | Yes | Parental assistance | Software | Complete telemedicine intervention (mixed) | |||||
| Xu et al [ ], 2021, China | Nurse and third-party health manager | Smartphone app | Smartphone app and smart wearable device | — | Yes | No | Yes | Yes | Yes | Independently | Software | Complete telemedicine intervention (mixed) | |||||
a Not reported.
The communication technologies used in the telemedicine interventions included in the studies took a variety of forms. Patients initiated communication with health care providers through different forms of telemedicine: smart wearable devices (6/20, 30%) [ 23 , 26 , 32 , 34 , 35 , 40 ], smartphone apps (5/20, 25%) [ 22 , 24 , 36 - 38 ], modem (3/20, 15%) [ 21 , 27 , 29 ], websites (3/20, 15%) [ 28 , 30 , 33 ], telephone (2/20, 10%) [ 31 , 39 ], and unclear (1/20, 5%) [ 25 ]. Health care providers initiated communication with patients through different forms of telemedicine: smartphone apps (5/20, 25%) [ 23 , 36 - 39 ], websites (3/20, 15%) [ 27 , 30 , 40 ], web conferences (3/20, 15%) [ 33 - 35 ], telephone (3/20, 15%) [ 21 , 28 , 31 ], SMS text messaging (3/20, 15%) [ 22 , 24 , 25 ], computer software (2/20, 10%) [ 26 , 29 ], or smart wearable devices (1/20, 5%) [ 32 ]. A total of 85% (17/20) of the studies mainly used various types of software [ 22 - 30 , 33 - 40 ], and 15% (3/20) of the studies used hardware [ 21 , 31 , 32 ].
In total, 45% (9/20) of the studies involved patients using telemedicine with parental assistance [ 26 , 27 , 31 , 33 - 37 , 40 ], and 30% (6/20) of the studies involved patients using telemedicine independently [ 23 - 25 , 28 , 32 , 38 ]. The form of intervention was complete telemedicine intervention in 60% (12/20) of the studies [ 21 , 24 , 27 , 30 - 34 , 36 - 38 , 40 ] and partial telemedicine intervention in 40% (8/20) of the studies [ 22 , 23 , 25 , 26 , 28 , 29 , 35 , 39 ]. The content of the telemedicine interventions in the studies included a variety of features: internet-based communication and follow-up (17/20, 85%) [ 21 - 24 , 26 , 28 , 29 , 31 - 40 ], medication dose adjustment (14/20, 70%) [ 22 , 24 , 26 - 29 , 31 , 32 , 34 - 37 , 39 , 40 ], basic health education (9/20, 45%) [ 21 , 22 , 24 , 30 , 31 , 33 , 35 , 37 , 38 ], diet guidance (9/20, 45%) [ 26 , 27 , 29 , 31 - 33 , 37 - 39 ], and physical exercise (6/20, 30%) [ 22 , 31 - 33 , 35 , 38 ]. A total of 55% (11/20) of the studies reported characteristics of the content of the intervention including at least 3 features [ 22 , 24 , 26 , 29 , 31 - 33 , 35 , 37 - 39 ]. No features of the content of the telemedicine interventions were reported in 5% (1/20) of the studies [ 25 ].
Risk of Bias
On the basis of the Cochrane Risk of Bias 2 tool, all studies except for 5% (1/20) with a high risk of bias [ 25 ] and 20% (4/20) with a low risk of bias [ 23 , 26 , 32 , 39 ] were found to have “some concerns” ( Figures 2 and 3 [ 21 - 40 ]). The greatest bias was found in the randomization process. Randomization was reported to be implemented in all studies, among which only 25% (5/20) of the studies explicitly described the randomization strategies and properly applied allocation concealment [ 23 , 26 , 30 , 32 , 39 ]. The other study [ 25 ] was rated as high risk because of baseline differences between intervention groups. No preregistration was reported in 45% (9/20) of the studies [ 21 , 24 , 27 - 29 , 31 , 33 , 34 , 40 ], and the risk of bias regarding the choice of reporting outcomes was rated as “some concerns.” One of the domains with the highest proportion of low risk of bias was “bias from missing outcome data.”

Meta-Analysis and Descriptive Analysis Results
A summary of the main results for the comparisons using the Grading of Recommendations, Assessment, Development, and Evaluations ratings is presented in Table 2 . Detailed meta-analytic forest plots on all outcomes and subgroups are shown in Figure 4 and Figure S1 in Multimedia Appendix 1 .
| Certainty assessment | Patients, n | Effect, absolute (95% CI; value) | Certainty | |||||||||||
| Studies, n (%) | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Telemedicine | Usual care | ||||||
| 20 (100) | Randomized trials | Serious | Not serious | Not serious | Serious | None | 822 | 822 | MD –0.22 (–0.33 to –0.10; <.001) | Low | ||||
| (impact of diabetes) | ||||||||||||||
| 2 (10) | Randomized trials | Serious | Not serious | Not serious | Very serious | None | 30 | 23 | MD 1.27 (–3.31 to 5.86; .59) | Very low | ||||
| 2 (10) | Randomized trials | Serious | Not serious | Not serious | Very serious | None | 30 | 23 | MD 0.58 (–2.59 to 3.66; .71) | Very low | ||||
| 2 (10) | Randomized trials | Serious | Serious | Not serious | Very serious | None | 30 | 23 | MD 3.27 (–12.53 to 19.08; .68) | Very low | ||||
| 3 (15) | Randomized trials | Serious | Not serious | Not serious | Serious | None | 165 | 160 | SMD –0.24 (–0.45 to –0.02; .04) | Low | ||||
| 3 (15) | Randomized trials | Serious | Serious | Not serious | Very serious | None | 96 | 91 | MD 0.54 (–0.72 to 1.8; .40) | Very low | ||||
| 4 (20) | Randomized trials | Serious | Serious | Not serious | Serious | None | 153 | 156 | MD 0.22 (–0.66 to 0.23; .49) | Very low | ||||
a HbA 1c : glycated hemoglobin.
b Downgraded for unclear or inadequate randomization process (15/20, 75% of the included studies). In a large number of studies, allocation was not adequately concealed due to the nature of the intervention.
c Although the Cochran Q test and Higgins I 2 test suggested a low heterogeneity, we chose not to downgrade for inconsistency as this was fully explained by the inclusion of 1 study.
d A total of 65% (13/20) of the studies had sample sizes of <50 in both arms.
e MD: mean difference.
f DQOLY: Diabetes Quality of Life for Youth.
g One of the studies had some concerns (a moderate risk of bias).
h Sample sizes for each arm of the included studies were <50.
i Significant heterogeneity.
j N-QOL: non–youth-specific quality of life.
k All 3 studies had some concerns.
l There was at least 1 study with a sample size of <50 in both arms.
m SMD: standardized mean difference.
n SMBG: self-monitoring of blood glucose.
o A total of 10% (2/20) of the studies had some concerns.
p One study had some concerns.

Effect of Telemedicine Interventions on HbA1c
The 20 studies, which reported HbA 1c levels at 3 to 50 months and examined 1704 participants, were included in the meta-analysis. Overall, telemedicine was found to reduce HbA 1c levels by 0.22 (95% CI –0.33 to –0.10; P <.001) at the end of the intervention. Furthermore, the heterogeneity of the effect size was confirmed as I 2 was 35% ( Q 19 =29.23; P =.06), suggesting heterogeneity of a low degree. Given the wide variety of technologies available for telemedicine, the heterogeneity of results is not surprising. No significant improvements were noted at the end of the 3- (MD –0.30, 95% CI –0.62 to 0.02; P =.07; n=4) or 12-month (MD –0.04, 95% CI –0.33 to 0.40; P =.85; n=2) follow-up; however, significant improvement was found at the end of the 6-month follow-up (MD –0.21, 95% CI –0.37 to –0.05; P =.01; n=8).
Effect of Telemedicine Interventions on Secondary Outcomes
We pooled the Diabetes Quality of Life for Youth (DQOLY) scores [ 51 , 52 ] from 10% (2/20) of the studies (n=53) [ 24 , 39 ], non–youth-specific quality of life (N-QOL; using diabetes quality of life [ 53 ] and health-related quality of life [ 54 ]) from 15% (3/20) of the studies (n=334) [ 35 , 38 , 40 ], daily frequency of SMBG from 15% (3/20) of the studies (n=187) [ 23 , 34 , 40 ], and incidence of hypoglycemia from 20% (4/20) of the studies (n=309) [ 23 , 26 , 32 , 38 ].
There was no significant effect size in secondary outcomes except for the N-QOL, with MD for DQOLY (DQOLY impact of diabetes subscale: MD 1.27, 95% CI –3.31 to 5.86, n=53, and I 2 =32.2%; DQOLY worries about diabetes subscale: MD 0.58, 95% CI –2.49 to 3.66, n=53, and I 2 =23.8%; DQOLY satisfaction with diabetes subscale: MD 3.27, 95% CI –12.53 to 19.08, n=53, and I 2 =75.6%), an SMD of −0.24 for the N-QOL (95% CI –0.45 to –0.02; n=334; I 2 =0%), an MD of 0.54 for daily frequency of SMBG (95% CI –0.72 to 1.80; n=187; I 2 =67.8%), and an SMD of −0.22 for incidence of hypoglycemia (95% CI –0.66 to 0.23; n=309; I 2 =73.7%).
Only 5% (1/20) of the studies [ 21 ] reported economic data. The difference in cost-effectiveness of care between the 2 groups was significant. The average cost per patient in the intervention group for the 6 months was US $163. The control group spent an average of US $246 to visit the clinic. If additional costs (average US $59), such as mileage, parking, meals, hotel stays, and babysitting, were included, the average cost of a clinic visit increased to US $305. This result shows that the telemedicine intervention was cost-effective, at least in the United States.
Subgroup Analysis of HbA1c
Our subgroup analysis based on study and intervention characteristics revealed that the subgroup differences that yielded statistical significance were publication date, communication forms (from patient to provider), and internet-based follow-up ( Table 3 ).
Regardless of age, intervention duration, and health care provider, HbA 1c levels significantly decreased in all studies after the telemedicine intervention.
| Characteristic and subgroup | Number of trials (number of participants) | Effect size, MD (95% CI) | (%) | value ( test) | Heterogeneity between groups | |||||||||
| 0.058 | ||||||||||||||
| Children | 2 (210) | –0.41 (–0.62 to –0.20) | 0 | .71 | ||||||||||
| Adolescents | 18 (1434) | –0.18 (–0.30 to –0.06) | 28.1 | .13 | ||||||||||
| 0.010 | ||||||||||||||
| 2010 and before | 5 (384) | 0.11 (–0.15 to 0.37) | 0 | .80 | ||||||||||
| After 2010 | 15 (1260) | –0.27 (–0.40 to –0.15) | 36.8 | .08 | ||||||||||
| 0.199 | ||||||||||||||
| <6 months | 8 (495) | –0.32 (–0.48 to –0.17) | 0 | .58 | ||||||||||
| At least 6 months | 12 (1149) | –0.18 (–0.33 to –0.03) | 43.2 | .06 | ||||||||||
| 0.884 | ||||||||||||||
| Professional diabetes care team | 9 (850) | –0.21 (–0.38 to –0.04) | 33.5 | .15 | ||||||||||
| No professional diabetes care team | 11 (794) | –0.23 (–0.40 to –0.06) | 40.7 | .08 | ||||||||||
| 0.426 | ||||||||||||||
| More than once a month | 5 (364) | –0.23 (–0.449 to –0.002) | 0 | .63 | ||||||||||
| Less than or equal to once a month | 10 (983) | –0.27 (–0.41 to –0.12) | 35.6 | .12 | ||||||||||
| Unclear | 5 (297) | –0.08 (–0.32 to 0.16) | 20.3 | .29 | ||||||||||
| 0.259 | ||||||||||||||
| Telephone | 3 (244) | –0.06 (–0.46 to 0.35) | 34.9 | .22 | ||||||||||
| SMS text messaging | 3 (229) | –0.10 (–0.35 to 0.15) | 45.4 | .16 | ||||||||||
| Smartphone app | 5 (440) | –0.37 (–0.53 to –0.21) | 0 | .62 | ||||||||||
| Computer software | 2 (211) | –0.12 (–0.59 to 0.35) | 39.6 | .20 | ||||||||||
| Website | 3 (127) | 0.00 (–0.36 to 0.36) | 0 | .63 | ||||||||||
| Web conference | 3 (330) | –0.34 (–0.65 to –0.02) | 49.9 | .14 | ||||||||||
| Smart wearable device | 1 (63) | −0.53 (−1.18 to 0.12) | — | — | ||||||||||
| 0.002 | ||||||||||||||
| Modem | 3 (209) | 0.11 (–0.26 to 0.48) | 0 | .76 | ||||||||||
| Smartphone app | 5 (453) | –0.37 (–0.51 to –0.22) | 0 | .96 | ||||||||||
| Smart wearable device | 6 (595) | –0.20 (–0.37 to –0.03) | 0 | .60 | ||||||||||
| Telephone | 2 (156) | –0.15 (–0.87 to 0.58) | 78.3 | .03 | ||||||||||
| Website | 3 (139) | –0.53 (–0.89 to –0.18) | 0 | .81 | ||||||||||
| Not reported | 1 (92) | 0.03 (–0.13 to 0.19) | — | — | ||||||||||
| 0.505 | ||||||||||||||
| Hardware | 3 (249) | –0.08 (–0.51 to 0.35) | 43.2 | .17 | ||||||||||
| Software | 17 (1395) | –0.23 (–0.36 to –0.11) | 34.8 | .08 | ||||||||||
| 0.534 | ||||||||||||||
| Independent use | 6 (374) | –0.11 (–0.31 to 0.09) | 0 | .53 | ||||||||||
| Parental assistance | 9 (899) | –0.24 (–0.41 to –0.07) | 44.1 | .07 | ||||||||||
| Unclear | 5 (371) | –0.26 (–0.47 to –0.04) | 0 | .53 | ||||||||||
| 0.206 | ||||||||||||||
| Complete telemedicine intervention | 12 (802) | –0.28 (–0.43 to –0.13) | 20.5 | .24 | ||||||||||
| Partial telemedicine intervention | 8 (842) | –0.14 (–0.29 to 0.01) | 29.4 | .19 | ||||||||||
| 0.002 | ||||||||||||||
| With feature | 17 (1460) | –0.27 (–0.38 to –0.17) | 8.9 | .35 | ||||||||||
| Without feature | 3 (184) | 0.03 (–0.12 to 0.18) | 0 | .64 | ||||||||||
| 0.577 | ||||||||||||||
| With feature | 14 (1267) | –0.25 (–0.37 to –0.12) | 20 | .24 | ||||||||||
| Without feature | 6 (377) | –0.17 (–0.41 to 0.07) | 37.4 | .16 | ||||||||||
| 0.823 | ||||||||||||||
| With feature | 6 (691) | –0.24 (–0.47 to 0.01) | 44.6 | .11 | ||||||||||
| Without feature | 14 (953) | –0.21 (–0.35 to –0.07) | 35.2 | .09 | ||||||||||
a MD: mean difference.
b Data synthesis is not possible with only one study.
Population of the Study
No statistically significant subgroup differences were identified in the subgroup analysis by age. A statistically significant decrease in HbA 1c levels was observed in subgroups of children (MD –0.41, 95% CI –0.62 to –0.20; P <.001) and adolescents (MD –0.18, 95% CI –0.30 to –0.06; P =.003). The children subgroup reported a higher MD than the adolescent subgroup.
Publication Date of the Studies
Subgroup analysis stratified by publication date demonstrated significant effectiveness of studies published after 2010 on glycemic control in children and adolescents with T1DM compared with those published before 2010. Moreover, a decrease in heterogeneity and statistically significant subgroup differences was found in the subgroup analysis based on publication date ( P =.01), which can explain the heterogeneity in overall effect on HbA 1c levels.
Duration of Telemedicine Interventions
We created 2 subgroups: interventions lasting <6 months and interventions lasting at least 6 months. The results revealed that telemedicine interventions lasting <6 months demonstrated a more significant reduction in HbA 1c levels (MD –0.32, 95% CI –0.48 to –0.17; P <.001).
Health Care Provider of Telemedicine Interventions
Subgroup analysis based on health care provider demonstrated significant effectiveness with or without the professional diabetes care team, and similar MDs were reported between the 2 groups (with care team: MD –0.21, 95% CI –0.38 to –0.04, and P =.02; without care team: MD –0.23, 95% CI –0.40 to –0.06, and P =.01).
Feedback Frequency of Telemedicine Interventions
Contrary to the nonsignificant overall effect of –0.01 on HbA 1c levels in 25% (5/20) of the studies with feedback (not reported), the overall effect in 25% (5/20) of the studies with feedback (more than once a month) was –0.23 (95% CI –0.449 to –0.002; P =.048), and the overall effect in 50% (10/20) of the studies with feedback (less than or equal to once a month) was –0.27 (95% CI –0.41 to –0.12; P< .001); the results of the study were statistically significant.
Communication Forms Between Patients and Providers
The choice of provider-to-patient communication forms—smartphone apps (MD –0.37, 95% CI –0.53 to –0.21; P <.001) and web conferences (MD –0.34, 95% CI –0.65 to –0.02; P =.04)—significantly influenced the effect of telemedicine on HbA 1c levels. In addition, the choice of patient-to-provider communication in the form of smartphone apps (MD –0.37, 95% CI –0.51 to –0.22; P <.001), smart wearable devices (MD –0.20, 95% CI –0.37 to –0.03; P =.02), and websites (MD –0.53, 95% CI –0.89 to –0.18; P =.003) had a significant impact on the effect on HbA 1c levels. A statistically significant subgroup difference was found in the subgroup analysis based on patient-to-provider communication forms ( P =.002).
Forms of Technology
Subgroup analysis by forms of technology showed that studies using software (MD –0.23, 95% CI –0.36 to –0.11; P <.001) had a significant effect on glycemic control in children and adolescents with T1DM compared with studies using only hardware (MD –0.08, 95% CI –0.51 to 0.35; P =.71).
Modes of Technology Use
The overall effect on HbA 1c levels in the 30% (6/20) of the studies with independent use of technology was –0.11 (95% CI –0.31 to 0.09; P =.27), whereas the overall effect on HbA 1c levels in the 45% (9/20) of the studies with parental assistance was –0.24 (95% CI –0.41 to –0.07; P <.001).
Forms of Telemedicine Interventions
Subgroup analysis based on the form of telemedicine intervention showed that complete telemedicine interventions (MD –0.28, 95% CI –0.43 to –0.13; P <.001) were better than partial telemedicine interventions (MD –0.14, 95% CI –0.29 to 0.01; P =.06).
Content of Telemedicine Interventions
Interventions with interactive communication and follow-up (MD –0.27, 95% CI –0.38 to –0.17; P <.001) and medication dose adjustment (MD –0.25, 95% CI –0.37 to –0.12; P <.001) were associated with a greater improvement in HbA 1c levels. However, interventions without a physical exercise feature also significantly influenced the effect of telemedicine on HbA 1c levels (MD –0.21, 95% CI –0.35 to –0.07; P =.004). Moreover, a decrease in heterogeneity and statistically significant subgroup differences was found in the subgroup analysis based on interactive communication and follow-up ( P =.002), which can also explain the heterogeneity in the overall effect on HbA 1c levels.
Sensitivity Analysis
Leave-one-out analysis was performed by removing each study, and there was no significant change in the effect size (Figure S2 in Multimedia Appendix 1 ). Accordingly, no individual study had a statistically significant effect on the overall result. However, inspection of the effect size identified one outlier study [ 25 ] with an effect size larger than that of the other studies. The exclusion of this study did not materially affect our results for the primary outcome, but it did reduce heterogeneity ( I 2 =9%; Q 18 =19.87; P =.34; fixed-effects model) and increase the impact of telemedicine (MD –0.26, 95% CI –0.36 to –0.17; P <.001).
Publication Bias
The contour funnel plot of HbA 1c levels was not obviously asymmetrical, consistent with publication bias (Figure S3 in Multimedia Appendix 1 ). We used the Egger regression test and Begg test to verify publication bias. The regression analysis bias estimate was insignificant (Egger test: bias=–1.02 and P= .32; Begg test: z= 0.16 and P= .87).
Meta-Regression
The results of the meta-regression are presented in Table S2 in Multimedia Appendix 1 . Meta-regression analysis showed that publication date ( P= .04) and the “Interactive follow-up” intervention characteristic ( P= .02) were moderating factors to explain the heterogeneity in this study.
Principal Findings
In this systematic review and meta-analysis of RCTs comparing telemedicine with usual care, the difference in HbA 1c levels was in favor of telemedicine (MD –0.22; P <.001). Sensitivity analysis showed low heterogeneity ( I 2 =35%; P =.06) and stability of the outliers. Subgroup analyses revealed that studies published after 2010, studies with <6 months of follow-up, studies in children with T1DM, studies in the form of smartphone apps (provider to patient) and websites (patient to provider) for communication, and studies with medication dose adjustment reported significantly larger effects of telemedicine. We were delighted to find that smartphone apps may be a particularly effective way of connecting providers and patients and that telemedicine improves quality of life for children and adolescents with T1DM (SMD –0.24, 95% CI –0.45 to –0.02; P =.04; I 2 =0%). However, there was no direct evidence that telemedicine could reduce the risk of hypoglycemia and improve SMBG. Our findings may help guide future clinical decision-making about the use of telemedicine for T1DM in children and adolescents.
Comparison With Prior Work
Our results showed that telemedicine interventions significantly reduced HbA 1c levels in children and adolescents with T1DM, which is similar to the results of previous meta-analyses in adults [ 20 , 55 - 57 ]. A recent study pointed out that a telemedicine intervention for HbA 1c in adults had a significant treatment effect [ 18 ]. In addition, Shulman et al [ 58 ] found no evidence for the effectiveness of telemedicine on HbA 1c levels in a 2010 meta-analysis specifically targeting T1DM in adolescents, which is consistent with the results of this study’s time-of-publication subgroup analysis. This suggested that telemedicine has evolved and improved rapidly over the past decade or so and is showing benefits for the treatment of children and adolescents with T1DM. In addition, the results of this study are contrary to the findings of the study by Lee et al [ 20 ], which did not find that telemedicine improved glycemic control in children and adolescents with T1DM by subgroup analysis.
Although improvements in the secondary outcomes of hypoglycemia risk and SMBG were not confirmed, it is encouraging to find that telemedicine improves quality of life in children and adolescents with T1DM. This is in contrast to previous studies with adolescents and children, where Shulman et al [ 58 ] did not find differences in quality of life between the telemedicine and control groups, and is also contrary to the results of previous studies [ 55 , 57 ] that did not restrict the type of diabetes and studies on T1DM [ 20 ] that did not restrict the population, which did not find a benefit of telemedicine in terms of quality of life.
In terms of studying the effect of follow-up time on HbA 1c levels, previous studies (not specifically for T1DM) [ 55 , 57 , 59 ] have shown that the effectiveness of telemedicine is higher when the intervention duration is at least 6 months. However, our findings are contrary to those presented in these studies. Our subgroup analysis showed a higher treatment effect in studies that lasted <6 months than in studies that lasted at least 6 months. This may be related to the “honeymoon” phase of T1DM. A “honeymoon” phase is a transient period of T1DM remission characterized by a significant reduction in insulin requirements and good glycemic control due to a temporary restoration of pancreatic β-cell function, which usually lasts for several months. The exact mechanisms are still uncertain, but one of the generally recognized mechanisms is that correction of “glucotoxicity” by exogenous insulin therapy leads to “β-cell rest” and β-cell recovery [ 60 ]. The concept of a “honeymoon” phase was first described by Jackson et al [ 61 ]. They observed a rapid decline in demand for exogenous insulin in children with diabetes after regular insulin treatment. In general, patients enter the “honeymoon” period approximately 3 months after starting insulin therapy, and it can last 6 to 9 months. Therefore, it is reasonable to speculate that, in T1DM studies with shorter intervention durations, patients are more likely to be influenced by the “honeymoon” period and, thus, show a better intervention effect. Future RCTs in this area should carefully consider the duration of telemedicine interventions in their design, which should be >6 months if possible, especially if it is not sufficiently known whether the enrolled group is in or has passed the “honeymoon” period. This is to minimize the effects of the intervention being influenced by the “honeymoon” period and improve the realism and reliability of the effectiveness of telemedicine interventions. In addition, this may be related to the fact that this study targeted children and adolescents with T1DM. An alternative explanation might be that patients become less responsive to monitoring prompts as the potential novelty of telemedicine interventions diminishes. This explanation is well recognized in the related area of activity tracking via smart wearable devices [ 62 ].
Our subgroup analysis results suggested differences between children and adolescents. Telemedicine interventions had a greater effect in children compared with adolescents. This contrasts with the findings of the study by Shulman et al [ 58 ], which showed no difference in HbA 1c levels between the adolescent and child subgroups at the end of the intervention. It may also be due to the use of different criteria for defining children in this study from those used by Shulman et al [ 58 ]. The most recent age criteria for children and adolescents used in this study limit the age of children to less than or equal to 10 years; however, based on speculation about the publication date of the study by Schulman et al [ 58 ], they may have defined the age of the children as older. Thus based on the age criteria of the present study, we anticipate that more child-related studies in the future may make this difference more apparent. By conducting subgroup analyses, we preliminarily excluded the influence of technology forms and use modes on this result. A total of 10% (2/20) of the studies were conducted on children, one using a hardware device independently [ 32 ] and the other using software with parental assistance [ 36 ]. However, we found that the studies on children were all complete telemedicine interventions. Subgroup analysis based on intervention form showed that complete telemedicine interventions were better than partial telemedicine interventions, which could explain the observed results. This finding is supported by the study by Chen et al [ 63 ], which found that a mixed complete telemedicine intervention was superior to a partial telemedicine intervention in reducing the incidence of pressure injury in patients with spinal cord injury. Another plausible explanation is that children’s blood glucose is more prone to fluctuations and a higher incidence of hypoglycemia compared to that of adolescents, which may lead to an exaggerated intervention effect. Although HbA 1c is the gold standard for long-term glycemic control, the use of HbA 1c alone to assess glycemic management in children can be misleading due to the magnitude of blood glucose fluctuations [ 64 ], and the pursuit of HbA 1c compliance can be accompanied by an increase in the frequency of hypoglycemia [ 65 , 66 ]. Hypoglycemia in children is a metabolic-endocrine emergency due to the potential for brain injury; permanent neurological sequelae; and, in rare cases, death [ 67 ]. Therefore, when assessing glycemic control in children, special attention should be paid to the incidence of hypoglycemia. We also found that telemedicine interventions with medication dose adjustment reported significant treatment effects in improving glycemic control in children and adolescents, consistent with the results of a study [ 55 ] on the effects of telemedicine on HbA 1c levels in patients with diabetes. Consequently, future well-designed studies should consider further enhancing insulin adjustment and monitoring in the intervention.
On the basis of the subgroup analysis by communication form, our results suggested that smartphone apps may be a very effective vehicle for linking intervention providers and patients, which can provide an intelligent management pathway for blood glucose in children and adolescents with T1DM. Nkhoma et al [ 68 ] also supported that smartphone apps improved glycemic control better than other tools. Moreover, the smartphone app studies included in this review (5/20, 25%) all evaluated the safety of apps and reported the incidence of adverse events such as hypoglycemia and diabetic ketoacidosis. Overall, smartphone apps are safe and do not increase the number of episodes of hypoglycemia [ 69 ]. Future studies could conduct an in-depth analysis of various types of smartphone apps in terms of core functionality (eg, health monitoring, smart health interventions and guidance, community interactions, and professional support), interface design and interaction experience, and dynamic sensing and self-adaptation (eg, automatically recommending personalized health plans based on the user’s basic information, such as age, gender, and body weight) to further improve the telemedicine intervention’s usability and effectiveness. This will enable children or adolescents with T1DM to benefit more from telemedicine.
Concerning cost-effectiveness, evidence is still lacking. Few studies included in this meta-analysis (1/20, 5%) discussed cost considerations, which is a common issue faced by telemedicine intervention studies. However, there are specific telemedicine cost analysis studies that may provide assistance with cost considerations. In a recently published study on the cost-effectiveness of telemedicine interventions, smartphone app, SMS text messaging, and website interventions were confirmed to be cost-effective without substantial differences among the different delivery modes [ 70 ]. A study by Elliott et al [ 71 ] showed that smart wearable devices increase short-term costs but their HbA 1c -lowering benefits will provide sufficient long-term health benefits and cost savings to justify the costs as long as the effects last into the medium term. The implementation of telemedicine services continues to be limited by cost and reimbursement barriers; future studies should increase transparency and conduct rigorous and in-depth cost-effectiveness analyses of the various types of telemedicine strategies to support T1DM management.
Practice, Policy, and Future Study
Our findings have potential ramifications for practice and policy. First, among studies evaluating the use of telemedicine interventions to improve care for children and adolescents with T1DM, we found that all (20/20, 100%) focused on HbA 1c , with only a small proportion of studies (9/20, 45%) reporting other outcomes such as quality of life and incidence of hypoglycemia. This prevents policy makers from considering the impact of interventions on outcomes other than HbA 1c when developing and implementing telemedicine interventions for this population. This situation may result in the health care system failing to respond to the needs of children and adolescents with T1DM and creates difficulties in tailoring telemedicine interventions to this population [ 72 ]. Focusing only on HbA 1c may, in turn, compromise the continuity of managed care for patients with T1DM. Therefore, we suggest that future studies add the assessment of other important outcomes such as quality of life, incidence of hypoglycemia, SMBG, and cost-effectiveness.
However, the importance of HbA 1c is undisputed, with findings published by the UK Prospective Diabetes Study as early as 2000 showing that a 1% reduction in mean HbA 1c levels was associated with a 21% reduction in diabetes-related deaths, a 14% reduction in the risk of myocardial infarction, and a 37% reduction in microvascular complications in patients with type 2 diabetes mellitus [ 73 ]. Results of a recent cross-sectional study of 156,090 children and adolescents with T1DM showed that the probability of diabetic retinopathy increased with increasing HbA 1c levels (adjusted odds ratio per 1 mmol/mol increase in HbA 1c levels 1.03, 95% CI 1.03-1.03; P <.001) [ 74 ]. Therefore, if telemedicine could be implemented in all children and adolescents with T1DM, it would help reduce the risk of macrovascular and microvascular complications, improve glycemic control, and enhance quality of life.
In light of the aforementioned, our findings suggest a promising application of telemedicine in the management of the disease in children and adolescents with T1DM, especially after several decades of development, during which telemedicine has shown many benefits for children and adolescents with T1DM. Future studies should carefully consider the various forms of interventions as well as the age group of the target population when tailoring telemedicine interventions for T1DM in adolescents and children, particularly with regard to the need for self-monitoring and recognition of hypoglycemia. Although the results of this study suggest that smartphone apps may be the best way to improve patients’ glycemic control, they may not be applicable to children aged <10 years. Taking China as an example, in addition to Chinese education policy discouraging the use of electronic devices in schools to minimize disruption and promote traditional teaching methods, children’s weaker self-control and potential addiction to gaming and entertainment, difficulties in parental supervision, and adverse effects on children’s face-to-face interactions and social skill development are important factors that make it difficult to apply this form of telemedicine.
Finally, this study also identified the lowest threshold of intervention duration intervals that may be able to safeguard the effectiveness of telemedicine interventions in children and adolescents with T1DM, making it necessary to conduct further studies with longer durations and larger cohort sizes in the future to determine the optimal intervals of intervention duration. Although this may be difficult; patients’ ability to improve their self-management of glycemia through telemedicine is a gradual process involving multiple factors, including patients’ learning ability, adaptability, acceptance of the technology, and the level of support from the health care team; and the time to achieve independent glycemic management may vary due to individual differences, the conduct of studies of longer durations is still very much appreciated.
Strengths and Limitations
This systematic review and meta-analysis has several strengths. To our knowledge, this is the first meta-analysis on telemedicine aimed at improving HbA 1c levels in children and adolescents. The substantial number of included RCTs and participants provided strong evidence for the clinical application of telemedicine for improving glycemic control in children and adolescents with T1DM. Second, we performed a relatively comprehensive subgroup analysis and confirmed that telemedicine may have the opposite effect in children and adolescents than in adults in terms of intervention duration. In addition, we undertook a comprehensive search of multiple databases and strictly adhered to methodological tools to report our research. Finally, we performed a leave-one-out sensitivity analysis, which allowed us to assess whether high-risk studies influenced the final results; however, excluding the high-risk study did not change the final results.
We also acknowledge that this meta-analysis has several limitations, mainly statistical assumptions such as deriving the mean and SD from the sample size, baseline, end point, and median, although these assumptions were robust in several sensitivity analyses. Second, data extraction could have included more baseline data from the study, such as medication use since diagnosis (total daily insulin dose, number of insulin injections per day, and insulin pump use), ethnicity, and nationality. Third, there was a certain degree of heterogeneity in the different types of telemedicine interventions. However, subgroup analysis should overcome this flaw. Fourth, only RCTs were included in this research; observational studies may yield pertinent insights for the correlation between telemedicine and HbA 1c levels. Fifth, most RCTs (15/20, 75%) did not explicitly report blinding or allocation concealment procedures because of intervention characteristic limitations, which would lead to performance and detection biases. Sixth, the precision of some secondary outcomes was relatively low because of the small number of relevant trials. More RCTs of high quality and with large sample sizes are needed for further validation. Finally, only articles published in English were reviewed, which would lead to potential selection bias, and therefore, the results’ generalizability may be limited.
Conclusions
Our systematic review and meta-analysis has shown that telemedicine is an efficacious and safe intervention approach. It can reduce HbA 1c levels and improve quality of life in children and adolescents with T1DM. Complete telemedicine interventions are better than partial telemedicine interventions. However, in accordance with the idea of providing health care from a distance, telemedicine should not be regarded as a uniform approach to medication or as an alternative to usual care but rather as a useful supplement to usual care to control HbA 1c levels and a potentially cost-effective mode. Given the potential benefits of telemedicine, such as greater access for remote populations or people with ambulatory restrictions, these findings may encourage further implementation of eHealth strategies for T1DM management, particularly as part of multifaceted interventions for integrated care of chronic diseases. The aforementioned conclusions need to be further verified in future studies. Meanwhile, researchers should develop higher-quality RCTs using large samples that focus on hard clinical outcomes, cost-effectiveness, and quality of life.
Acknowledgments
The authors are sincerely grateful for funding from Fujian Provincial Health Science and Technology Program project funding scheme for 2021 by the Fujian Provincial Health Commission (grant 2021CXB007). They thank all the individuals who contributed to this study.
Authors' Contributions
KZ and QW contributed to the study concept and design. KZ drafted the manuscript. QH helped draft the manuscript. KZ, CL, and QH assessed the risk of bias. QH and QW assessed the quality of evidence of each study. KZ and CL independently extracted the data for analysis. KZ, QZ, DX, CX, MZ, and RL were involved in discussing earlier versions of the text. All authors participated in the study design and read and approved the final manuscript.
Conflicts of Interest
None declared.
Search strategy, funnel plot for primary outcome, forest plots of the subgroups and the secondary outcomes, summary of meta-regression results, and sensitivity analysis.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 checklist.
- Sperling MA, Laffel LM. Current management of glycemia in children with type 1 diabetes mellitus. N Engl J Med. Mar 24, 2022;386(12):1155-1164. [ CrossRef ] [ Medline ]
- DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. Jun 16, 2018;391(10138):2449-2462. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Magliano DJ, Boyko EJ. IDF diabetes atlas: diabetes around the world in 2021. International Diabetes Federation. URL: https://diabetesatlas.org/atlas/tenth-edition/ [accessed 2024-04-29]
- Gregory JW, Cameron FJ, Joshi K, Eiswirth M, Garrett C, Garvey K, et al. ISPAD clinical practice consensus guidelines 2022: diabetes in adolescence. Pediatr Diabetes. Nov 2022;23(7):857-871. [ FREE Full text ] [ CrossRef ] [ Medline ]
- von Scholten BJ, Kreiner FF, Gough SC, von Herrath M. Current and future therapies for type 1 diabetes. Diabetologia. May 2021;64(5):1037-1048. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Bjornstad P, Donaghue KC, Maahs DM. Macrovascular disease and risk factors in youth with type 1 diabetes: time to be more attentive to treatment? Lancet Diabetes Endocrinol. Oct 2018;6(10):809-820. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Marcovecchio ML, Dalton RN, Daneman D, Deanfield J, Jones TW, Neil HA, et al. Adolescent type 1 Diabetes cardio-renal Intervention Trial (AdDIT) study group. A new strategy for vascular complications in young people with type 1 diabetes mellitus. Nat Rev Endocrinol. Jul 2019;15(7):429-435. [ CrossRef ] [ Medline ]
- Katsarou A, Gudbjörnsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, et al. Type 1 diabetes mellitus. Nat Rev Dis Primers. Mar 30, 2017;3:17016. [ CrossRef ] [ Medline ]
- Teo E, Hassan N, Tam W, Koh S. Effectiveness of continuous glucose monitoring in maintaining glycaemic control among people with type 1 diabetes mellitus: a systematic review of randomised controlled trials and meta-analysis. Diabetologia. Apr 2022;65(4):604-619. [ CrossRef ] [ Medline ]
- Global strategy on digital health 2020-2025. World Health Organization. URL: https://www.who.int/publications/i/item/9789240020924 [accessed 2024-04-29]
- Chan RJ, Crichton M, Crawford-Williams F, Agbejule OA, Yu K, Hart NH, et al. Multinational Association of Supportive Care in Cancer (MASCC) Survivorship Study Group. The efficacy, challenges, and facilitators of telemedicine in post-treatment cancer survivorship care: an overview of systematic reviews. Ann Oncol. Dec 2021;32(12):1552-1570. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Cunha AS, Pedro AR, Cordeiro JV. Facilitators of and barriers to accessing hospital medical specialty telemedicine consultations during the COVID-19 pandemic: systematic review. J Med Internet Res. Jul 10, 2023;25:e44188. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Anderson K, Francis T, Ibanez-Carrasco F, Globerman J. Physician's perceptions of telemedicine in HIV care provision: a cross-sectional web-based survey. JMIR Public Health Surveill. May 30, 2017;3(2):e31. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Sengupta A, Pettigrew S, Jenkins CR. Telemedicine in specialist outpatient care during COVID-19: a qualitative study. Intern Med J. Jan 2024;54(1):54-61. [ CrossRef ] [ Medline ]
- Ruiz de Adana MS, Alhambra-Expósito MR, Muñoz-Garach A, Gonzalez-Molero I, Colomo N, Torres-Barea I, et al. Diabetes Group of SAEDYN (Andalusian Society of Endocrinology‚ Diabetes‚ Nutrition). Randomized study to evaluate the impact of telemedicine care in patients with type 1 diabetes with multiple doses of insulin and suboptimal HbA in Andalusia (Spain): PLATEDIAN study. Diabetes Care. Feb 2020;43(2):337-342. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Molavynejad S, Miladinia M, Jahangiri M. A randomized trial of comparing video telecare education vs. in-person education on dietary regimen compliance in patients with type 2 diabetes mellitus: a support for clinical telehealth Providers. BMC Endocr Disord. May 02, 2022;22(1):116. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Bisno DI, Reid MW, Fogel JL, Pyatak EA, Majidi S, Raymond JK. Virtual group appointments reduce distress and improve care management in young adults with type 1 diabetes. J Diabetes Sci Technol. Nov 2022;16(6):1419-1427. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Udsen FW, Hangaard S, Bender C, Andersen J, Kronborg T, Vestergaard P, et al. The effectiveness of telemedicine solutions in type 1 diabetes management: a systematic review and meta-analysis. J Diabetes Sci Technol. May 2023;17(3):782-793. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Eberle C, Stichling S. Clinical improvements by telemedicine interventions managing type 1 and type 2 diabetes: systematic meta-review. J Med Internet Res. Feb 19, 2021;23(2):e23244. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lee SW, Ooi L, Lai YK. Telemedicine for the management of glycemic control and clinical outcomes of type 1 diabetes mellitus: a systematic review and meta-analysis of randomized controlled studies. Front Pharmacol. 2017;8:330. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Chase HP, Pearson JA, Wightman C, Roberts MD, Oderberg AD, Garg SK. Modem transmission of glucose values reduces the costs and need for clinic visits. Diabetes Care. May 2003;26(5):1475-1479. [ CrossRef ] [ Medline ]
- Gandrud L, Altan A, Buzinec P, Hemphill J, Chatterton J, Kelley T, et al. Intensive remote monitoring versus conventional care in type 1 diabetes: a randomized controlled trial. Pediatr Diabetes (Forthcoming). Feb 21, 2018. [ CrossRef ] [ Medline ]
- Goyal S, Nunn CA, Rotondi M, Couperthwaite AB, Reiser S, Simone A, et al. A mobile app for the self-management of type 1 diabetes among adolescents: a randomized controlled trial. JMIR Mhealth Uhealth. Jun 19, 2017;5(6):e82. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Han Y, Faulkner MS, Fritz H, Fadoju D, Muir A, Abowd GD, et al. A pilot randomized trial of text-messaging for symptom awareness and diabetes knowledge in adolescents with type 1 diabetes. J Pediatr Nurs. Nov 2015;30(6):850-861. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ibrahim N, Treluyer JM, Briand N, Godot C, Polak M, Beltrand J. Text message reminders for adolescents with poorly controlled type 1 diabetes: a randomized controlled trial. PLoS One. 2021;16(3):e0248549. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kowalska A, Piechowiak K, Ramotowska A, Szypowska A. Impact of ELKa, the electronic device for prandial insulin dose calculation, on metabolic control in children and adolescents with type 1 diabetes mellitus: a randomized controlled trial. J Diabetes Res. 2017;2017:1708148. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kumar VS, Wentzell KJ, Mikkelsen T, Pentland A, Laffel LM. The DAILY (daily automated intensive log for youth) trial: a wireless, portable system to improve adherence and glycemic control in youth with diabetes. Diabetes Technol Ther. Aug 2004;6(4):445-453. [ CrossRef ] [ Medline ]
- Landau Z, Mazor-Aronovitch K, Boaz M, Blaychfeld-Magnazi M, Graph-Barel C, Levek-Motola N, et al. The effectiveness of internet-based blood glucose monitoring system on improving diabetes control in adolescents with type 1 diabetes. Pediatr Diabetes. Mar 2012;13(2):203-207. [ CrossRef ] [ Medline ]
- Marrero DG, Vandagriff JL, Kronz K, Fineberg NS, Golden MP, Gray D, et al. Using telecommunication technology to manage children with diabetes: the computer-linked outpatient clinic (CLOC) study. Diabetes Educ. 1995;21(4):313-319. [ CrossRef ] [ Medline ]
- Mulvaney SA, Rothman RL, Wallston KA, Lybarger C, Dietrich MS. An internet-based program to improve self-management in adolescents with type 1 diabetes. Diabetes Care. Mar 2010;33(3):602-604. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Nunn E, King B, Smart C, Anderson D. A randomized controlled trial of telephone calls to young patients with poorly controlled type 1 diabetes. Pediatr Diabetes. Oct 2006;7(5):254-259. [ CrossRef ] [ Medline ]
- Raviteja KV, Kumar R, Dayal D, Sachdeva N. Clinical efficacy of professional continuous glucose monitoring in improving glycemic control among children with type 1 diabetes mellitus: an open-label randomized control trial. Sci Rep. Apr 16, 2019;9(1):6120. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Schiaffini R, Tagliente I, Carducci C, Ullmann N, Ciampalini P, Lorubbio A, et al. Impact of long-term use of eHealth systems in adolescents with type 1 diabetes treated with sensor-augmented pump therapy. J Telemed Telecare. Jul 2016;22(5):277-281. [ CrossRef ] [ Medline ]
- Stanger C, Lansing AH, Scherer E, Budney A, Christiano AS, Casella SJ. A web-delivered multicomponent intervention for adolescents with poorly controlled type 1 diabetes: a pilot randomized controlled trial. Ann Behav Med. Nov 12, 2018;52(12):1010-1022. [ FREE Full text ] [ CrossRef ] [ Medline ]
- von Sengbusch S, Eisemann N, Mueller-Godeffroy E, Lange K, Doerdelmann J, Erdem A, et al. Outcomes of monthly video consultations as an add-on to regular care for children with type 1 diabetes: a 6-month quasi-randomized clinical trial followed by an extension phase. Pediatr Diabetes. Dec 2020;21(8):1502-1515. [ CrossRef ] [ Medline ]
- Ware J, Allen JM, Boughton CK, Wilinska ME, Hartnell S, Thankamony A, et al. KidsAP Consortium. Randomized trial of closed-loop control in very young children with type 1 diabetes. N Engl J Med. Jan 20, 2022;386(3):209-219. [ CrossRef ] [ Medline ]
- Ware J, Boughton CK, Allen JM, Wilinska ME, Tauschmann M, Denvir L, et al. DAN05 Consortium. Cambridge hybrid closed-loop algorithm in children and adolescents with type 1 diabetes: a multicentre 6-month randomised controlled trial. Lancet Digit Health. Apr 2022;4(4):e245-e255. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Xu Y, Xu L, Zhao W, Li Q, Li M, Lu W, et al. Effectiveness of a WeChat combined continuous flash glucose monitoring system on glycemic control in juvenile type 1 diabetes mellitus management: randomized controlled trial. Diabetes Metab Syndr Obes. 2021;14:1085-1094. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Klee P, Bussien C, Castellsague M, Combescure C, Dirlewanger M, Girardin C, et al. An intervention by a patient-designed do-it-yourself mobile device app reduces HbA1c in children and adolescents with type 1 diabetes: a randomized double-crossover study. Diabetes Technol Ther. Dec 2018;20(12):797-805. [ CrossRef ] [ Medline ]
- Shalitin S, Ben-Ari T, Yackobovitch-Gavan M, Tenenbaum A, Lebenthal Y, de Vries L, et al. Using the Internet-based upload blood glucose monitoring and therapy management system in patients with type 1 diabetes. Acta Diabetol. Apr 2014;51(2):247-256. [ CrossRef ] [ Medline ]
- Campbell F, Biggs K, Aldiss SK, O'Neill PM, Clowes M, McDonagh J, et al. Transition of care for adolescents from paediatric services to adult health services. Cochrane Database Syst Rev. Apr 29, 2016;4(4):CD009794. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. Aug 28, 2019;366:l4898. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Schünemann HJ, Mustafa RA, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE Working Group. GRADE guidelines: 21 part 2. Test accuracy: inconsistency, imprecision, publication bias, and other domains for rating the certainty of evidence and presenting it in evidence profiles and summary of findings tables. J Clin Epidemiol. Jun 2020;122:142-152. [ CrossRef ] [ Medline ]
- Bayoumy K, Gaber M, Elshafeey A, Mhaimeed O, Dineen EH, Marvel FA, et al. Smart wearable devices in cardiovascular care: where we are and how to move forward. Nat Rev Cardiol. Aug 2021;18(8):581-599. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3. Hoboken, NJ. John Wiley & Sons; 2019.
- Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. Jun 2018;27(6):1785-1805. [ CrossRef ] [ Medline ]
- Shi J, Luo D, Weng H, Zeng XT, Lin L, Chu H, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. Sep 2020;11(5):641-654. [ CrossRef ] [ Medline ]
- Littell JH, Corcoran J, Pillai V. Systematic Reviews and Meta-Analysis. New York, NY. Oxford University Press; 2008.
- Mavridis D, Salanti G. How to assess publication bias: funnel plot, trim-and-fill method and selection models. Evid Based Ment Health. Feb 2014;17(1):30. [ CrossRef ] [ Medline ]
- Fu R, Gartlehner G, Grant M, Shamliyan T, Sedrakyan A, Wilt TJ, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the effective health care program. J Clin Epidemiol. Nov 2011;64(11):1187-1197. [ CrossRef ] [ Medline ]
- Ingersoll GM, Marrero DG. A modified quality-of-life measure for youths: psychometric properties. Diabetes Educ. 1991;17(2):114-118. [ CrossRef ] [ Medline ]
- Skinner TC, Hoey H, McGee HM, Skovlund SE, Hvidøre Study Group on Childhood Diabetes. A short form of the diabetes quality of life for youth questionnaire: exploratory and confirmatory analysis in a sample of 2,077 young people with type 1 diabetes mellitus. Diabetologia. Apr 2006;49(4):621-628. [ CrossRef ] [ Medline ]
- The DCCT Research Group. Reliability and validity of a diabetes quality-of-life measure for the diabetes control and complications trial (DCCT). Diabetes Care. Oct 1988;11(9):725-732. [ CrossRef ] [ Medline ]
- Ravens-Sieberer U, Bullinger M. Assessing health-related quality of life in chronically ill children with the German KINDL: first psychometric and content analytical results. Qual Life Res. Jul 1998;7(5):399-407. [ CrossRef ] [ Medline ]
- Faruque LI, Wiebe N, Ehteshami-Afshar A, Liu Y, Dianati-Maleki N, Hemmelgarn BR, et al. Alberta Kidney Disease Network. Effect of telemedicine on glycated hemoglobin in diabetes: a systematic review and meta-analysis of randomized trials. CMAJ. Mar 06, 2017;189(9):E341-E364. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Wang X, Shu W, Du J, Du M, Wang P, Xue M, et al. Mobile health in the management of type 1 diabetes: a systematic review and meta-analysis. BMC Endocr Disord. Feb 13, 2019;19(1):21. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Correia JC, Meraj H, Teoh SH, Waqas A, Ahmad M, Lapão LV, et al. Telemedicine to deliver diabetes care in low- and middle-income countries: a systematic review and meta-analysis. Bull World Health Organ. Mar 01, 2021;99(3):209-19B. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Shulman RM, O'Gorman CS, Palmert MR. The impact of telemedicine interventions involving routine transmission of blood glucose data with clinician feedback on metabolic control in youth with type 1 diabetes: a systematic review and meta-analysis. Int J Pediatr Endocrinol. 2010;2010:536957. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Tchero H, Kangambega P, Briatte C, Brunet-Houdard S, Retali GR, Rusch E. Clinical effectiveness of telemedicine in diabetes mellitus: a meta-analysis of 42 randomized controlled trials. Telemed J E Health. Jul 2019;25(7):569-583. [ CrossRef ] [ Medline ]
- Zhong T, Tang R, Gong S, Li J, Li X, Zhou Z. The remission phase in type 1 diabetes: changing epidemiology, definitions, and emerging immuno-metabolic mechanisms. Diabetes Metab Res Rev. Feb 2020;36(2):e3207. [ CrossRef ] [ Medline ]
- Jackson RL, Boyd JB, Smith TE. Stabilization of the diabetic child. Arch Pediatr Adolesc Med. Feb 01, 1940;59(2):332-341. [ FREE Full text ] [ CrossRef ]
- Shin G, Feng Y, Jarrahi MH, Gafinowitz N. Beyond novelty effect: a mixed-methods exploration into the motivation for long-term activity tracker use. JAMIA Open. Apr 2019;2(1):62-72. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Chen G, Wang T, Zhong L, He X, Huang C, Wang Y, et al. Telemedicine for preventing and treating pressure injury after spinal cord injury: systematic review and meta-analysis. J Med Internet Res. Sep 07, 2022;24(9):e37618. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA (1c) alone to assess glycemic control can be misleading. Diabetes Care. Aug 2017;40(8):994-999. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Gimenez M, Tannen AJ, Reddy M, Moscardo V, Conget I, Oliver N. Revisiting the relationships between measures of glycemic control and hypoglycemia in continuous glucose monitoring data sets. Diabetes Care. Feb 2018;41(2):326-332. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Seyed Ahmadi S, Westman K, Pivodic A, Ólafsdóttir AF, Dahlqvist S, Hirsch IB, et al. The association between HbA (1c) and time in hypoglycemia during CGM and self-monitoring of blood glucose in people with type 1 diabetes and multiple daily insulin injections: a randomized clinical trial (GOLD-4). Diabetes Care. Sep 2020;43(9):2017-2024. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Quarta A, Iannucci D, Guarino M, Blasetti A, Chiarelli F. Hypoglycemia in children: major endocrine-metabolic causes and novel therapeutic perspectives. Nutrients. Aug 11, 2023;15(16):3544. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Nkhoma DE, Soko CJ, Bowrin P, Manga YB, Greenfield D, Househ M, et al. Digital interventions self-management education for type 1 and 2 diabetes: a systematic review and meta-analysis. Comput Methods Programs Biomed. Oct 2021;210:106370. [ CrossRef ] [ Medline ]
- Pi L, Shi X, Wang Z, Zhou Z. Effect of smartphone apps on glycemic control in young patients with type 1 diabetes: a meta-analysis. Front Public Health. 2023;11:1074946. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Willems R, Annemans L, Siopis G, Moschonis G, Vedanthan R, Jung J, et al. DigiCare 4You. Cost effectiveness review of text messaging, smartphone application, and website interventions targeting T2DM or hypertension. NPJ Digit Med. Aug 18, 2023;6(1):150. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Elliott RA, Rogers G, Evans ML, Neupane S, Rayman G, Lumley S, et al. FLASH-UK Trial Study Group. Estimating the cost-effectiveness of intermittently scanned continuous glucose monitoring in adults with type 1 diabetes in England. Diabet Med. Mar 2024;41(3):e15232. [ CrossRef ] [ Medline ]
- Zafra-Tanaka JH, Beran D, Bernabe-Ortiz A. Health system responses for type 1 diabetes: a scoping review. Diabet Med. Jul 2022;39(7):e14805. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. Aug 12, 2000;321(7258):405-412. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Bratina N, Auzanneau M, Birkebaek N, de Beaufort C, Cherubini V, Craig ME, et al. Australasian Diabetes Data Network (ADDN) Study Group‚ the Prospective Diabetes Follow-up Registry (DPV) initiative‚ Danish National Diabetes Registry (DanDiabKids)‚ National Pediatric Diabetes Audit (NPDA)‚ Region Marche Registry for Diabetes‚ Diabeter Diabetes Database‚ Slovenian Childhood Diabetes Registry‚ SEARCH for Diabetes in Youth Study. Differences in retinopathy prevalence and associated risk factors across 11 countries in three continents: a cross-sectional study of 156,090 children and adolescents with type 1 diabetes. Pediatr Diabetes. Dec 2022;23(8):1656-1664. [ FREE Full text ] [ CrossRef ] [ Medline ]
Abbreviations
| Diabetes Quality of Life for Youth |
| : glycated hemoglobin |
| mean difference |
| non–youth-specific quality of life |
| Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| randomized controlled trial |
| self-monitoring of blood glucose |
| standardized mean difference |
| type 1 diabetes mellitus |
Edited by T Leung, T de Azevedo Cardoso; submitted 02.08.23; peer-reviewed by A Cunha, X Shi, A Zhang; comments to author 02.02.24; revised version received 28.02.24; accepted 21.05.24; published 09.07.24.
©Kun Zhang, Qiyuan Huang, Qiaosong Wang, Chengyang Li, Qirong Zheng, Zhuoyue Li, Dan Xu, Cuiling Xie, Mingqi Zhang, Rongjin Lin. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 09.07.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research (ISSN 1438-8871), is properly cited. The complete bibliographic information, a link to the original publication on https://www.jmir.org/, as well as this copyright and license information must be included.

Amid rise in childhood diabetes, man describes how to 'thrive' with disease

Data released by the Centers for Disease Control and Prevention earlier this year shows the U.S. is projected to have a significant increase in children diagnosed with diabetes.
According to the CDC, between now and 2060, the number of children with Type 2 diabetes is projected to increase by 700%, while cases of youths with Type 1 diabetes are expected to increase by 65%. While Type 2 diabetes can be delayed or prevented by diet and exercise, there is no known way to prevent Type 1 diabetes, the CDC said.
Author Ben Milsom released a book titled "The Fearless Diabetic" that millions of U.S. families can relate to. Milsom was diagnosed with Type 1 diabetes in 1997. He says his book "offers a real-life look at what it’s like to live with and thrive with this challenging disease."
While family history is considered a risk factor for Type 1 diabetes, Milsom said he had no known family history of the disease.
RELATED STORY | Popular diet and diabetes drugs in short supply as demand surges
"I had life in the palm of my hands, and then all of a sudden, I started losing weight, I started getting very sick, and I knew something was wrong," Milsom said about when he was diagnosed at age 17. "No one in my family had the disease, so I knew I had to go to the doctor and get it checked out. Whenever I went to the doctor, I told them my symptoms, and it was very apparent that Type 1 diabetes was going to be in my future."
Milsom has gone on to have a successful career in sports, working in the NHL, NBA and NFL. One night, however, showed the perils of working as a Type 1 diabetic.
Driving home after working a late basketball game, Milsom blacked out behind the wheel. Police arrested him as officers believed he was driving under the influence. Milsom said he was fortunate not to lose his job, but the incident prompted him to bring awareness to what triggered his blackout.
"That was before I had an insulin pump," he said. "Now I have an insulin pump with a continuous glucose monitor that tells me what my blood sugar is. It goes to my Apple Watch, so I know 24/7 just by looking at my watch what my blood sugar is. But that's just the advances in technology and how great it's become. If I would have had that technology, I could have avoided that entire situation."
RELATED STORY | Weight loss drugs not just being used by those to treat chronic conditions, survey finds
Milsom noted the battles that both Type 1 and Type 2 diabetics face.
"Both these Type 1 and Type 2 are relentless," he said. "They never stop. It's a 24/7 number check, and it's something we have to manage, and we have to fight every day and make sure that we help others that are going through some of the things that happen with other Type 1s."
Common symptoms for Type 1 diabetes
The CDC said people should consider seeing a doctor to have their blood sugar checked if they:
- Urinate a lot, often at night.
- Are very thirsty.
- Lose weight without trying.
- Are very hungry.
- Have blurry vision.
- Have numb or tingling hands or feet.
- Feel very tired.
- Have very dry skin.
- Have sores that heal slowly.
- Have more infections than usual.
Most Recent

Forget European summer, this US city can compete with any overseas, survey says

Hunter Biden drops request for new federal gun trial

'The Devil Wears Prada' sequel is reportedly in development at Disney

Mounjaro or Ozempic: Which is better for weight loss?

Health officials in Colorado confirm a rare case of human plague

Are your tampons harming you? Study finds 16 metals in widely available brands

Watch Scripps News
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Impact of type 1 diabetes mellitus, glucose levels, and glycemic control on sleep in children and adolescents: a case-control study
Affiliations.
- 1 Department of Women's and Children's Health, University of Otago, Dunedin, New Zealand.
- 2 Department of Paediatrics and Child Health, University of Otago, Wellington, New Zealand.
- 3 Department of Human Nutrition, University of Otago, Dunedin, New Zealand.
- 4 WellSleep Investigation Centre, Department of Medicine, University of Otago, Wellington, New Zealand and.
- 5 Paediatric Endocrinology, Southern District Health Board, Dunedin, New Zealand.
- PMID: 31583407
- DOI: 10.1093/sleep/zsz226
Study objectives: To assess differences in habitual sleep patterns and sleep states between children and adolescents with type 1 diabetes mellitus (T1DM) and control subjects, and to explore the relationships between sleep, glucose levels, and glycemic control.
Methods: Participants included 82 children (5-18 years); 41 with T1DM (cases), and 41 healthy control subjects group matched for age and sex. Sleep was measured by 7-day actigraphy and single-night home-based polysomnography (PSG) recordings. Hemoglobin A1c (HbA1c) and 7 days of continuous glucose monitoring (CGM) data were collected in cases. Regression analyses were used to model all within- and between-group comparisons adjusted for age, sex, and BMI z-scores.
Results: There were no significant differences in sleep duration, efficiency, or awakenings as measured by actigraphy and PSG between cases and controls, nor sleep states measured by PSG. However, cases had significantly later sleep onset and offset than controls (both p < 0.05), partially moderated by age. Cases with suboptimal glycemic control (HbA1c ≥ 58 mmol/mol [≥7.5%]) had significantly shorter actigraphy-derived total sleep time (TST) (mean difference = -40 minutes; 95% confidence interval = -77, -3), with similar differences in TST measured by PSG. Cases with mean CGM glucose levels ≥10 mmol/L (≥180 mg/dL) on PSG night had significantly more stage N3 (%) sleep and less stage REM (%) sleep (both p < 0.05).
Conclusions: Short- and long-term suboptimal glycemic control in T1DM children appears to be associated with sleep alterations. Pediatric diabetes care teams should be aware of potential interrelationships between sleep and T1DM, including management and glycemic control.
Keywords: actigraphy; continuous glucose monitoring; glycemic control; glycemic variability; hyperglycemia; polysomnography; sleep state architecture.
© Sleep Research Society 2019. Published by Oxford University Press on behalf of the Sleep Research Society. All rights reserved. For permissions, please e-mail [email protected].
PubMed Disclaimer
Similar articles
- Association between sleep variability and time in range of glucose levels in patients with type 1 diabetes: Cross-sectional study. Reutrakul S, Irsheed GA, Park M, Steffen AD, Burke L, Pratuangtham S, Baron KG, Duffecy J, Perez R, Quinn L, Withington MHC, Saleh AH, Loiacono B, Mihailescu D, Martyn-Nemeth P. Reutrakul S, et al. Sleep Health. 2023 Dec;9(6):968-976. doi: 10.1016/j.sleh.2023.07.007. Epub 2023 Sep 13. Sleep Health. 2023. PMID: 37709596
- Adherence as a Predictor of Glycemic Control Among Adolescents With Type 1 Diabetes: A Retrospective Study Using Real-world Evidence. Ibrahim SA, El Hajj MS, Owusu YB, Al-Khaja M, Khalifa A, Ahmed D, Awaisu A. Ibrahim SA, et al. Clin Ther. 2022 Oct;44(10):1380-1392. doi: 10.1016/j.clinthera.2022.09.003. Epub 2022 Oct 1. Clin Ther. 2022. PMID: 36192263
- Sleep and Type 1 Diabetes Mellitus Management Among Children, Adolescents, and Emerging Young Adults: A Systematic Review. Ji X, Wang Y, Saylor J. Ji X, et al. J Pediatr Nurs. 2021 Nov-Dec;61:245-253. doi: 10.1016/j.pedn.2021.06.010. Epub 2021 Jun 26. J Pediatr Nurs. 2021. PMID: 34182231 Review.
- Sleep, glucose, and daytime functioning in youth with type 1 diabetes. Perfect MM, Patel PG, Scott RE, Wheeler MD, Patel C, Griffin K, Sorensen ST, Goodwin JL, Quan SF. Perfect MM, et al. Sleep. 2012 Jan 1;35(1):81-8. doi: 10.5665/sleep.1590. Sleep. 2012. PMID: 22215921 Free PMC article.
- Efficacy and safety comparison of continuous glucose monitoring and self-monitoring of blood glucose in type 1 diabetes: systematic review and meta-analysis. Wojciechowski P, Ryś P, Lipowska A, Gawęska M, Małecki MT. Wojciechowski P, et al. Pol Arch Med Wewn. 2011 Oct;121(10):333-43. Pol Arch Med Wewn. 2011. PMID: 22045094 Review.
- Longitudinal relations of sleep quality with depressive symptoms, diabetes distress and self-efficacy in young people with type 1 diabetes. Nefs G, Feinn R, Chang AM, Wagner J. Nefs G, et al. J Psychosom Res. 2023 Oct;173:111457. doi: 10.1016/j.jpsychores.2023.111457. Epub 2023 Aug 10. J Psychosom Res. 2023. PMID: 37634323
- 24-Hour Movement Behaviours (Physical Activity, Sedentary Behaviour and Sleep) Association with Glycaemic Control and Psychosocial Outcomes in Adolescents with Type 1 Diabetes: A Systematic Review of Quantitative and Qualitative Studies. Patience M, Janssen X, Kirk A, McCrory S, Russell E, Hodgson W, Crawford M. Patience M, et al. Int J Environ Res Public Health. 2023 Feb 28;20(5):4363. doi: 10.3390/ijerph20054363. Int J Environ Res Public Health. 2023. PMID: 36901373 Free PMC article. Review.
- The impact of fear of hypoglycaemia on sleep in adolescents with type 1 diabetes. Hitt TA, Hershey JA, Olivos-Stewart D, Forth E, Stuart F, Garren P, Mitchell J, Hawkes CP, Willi SM, Gettings JM. Hitt TA, et al. Diabet Med. 2023 May;40(5):e15066. doi: 10.1111/dme.15066. Epub 2023 Feb 23. Diabet Med. 2023. PMID: 36786042 Free PMC article.
- Associations between Sleep Characteristics and Cardiovascular Risk Factors in Adolescents Living with Type 1 Diabetes. Wu N, Jamnik VK, Koehle MS, Guan Y, Li Y, Kaufman K, Warburton DER. Wu N, et al. J Clin Med. 2022 Sep 8;11(18):5295. doi: 10.3390/jcm11185295. J Clin Med. 2022. PMID: 36142941 Free PMC article.
- Glucose and unstructured physical activity coupling during sleep and wake in young adults with type 1 diabetes. Griggs S, Barbato E, Hernandez E, Gupta D, Margevicius S, Grey M, Hickman RL Jr. Griggs S, et al. Sci Rep. 2022 Apr 6;12(1):5790. doi: 10.1038/s41598-022-09728-2. Sci Rep. 2022. PMID: 35388088 Free PMC article.
Publication types
- Search in MeSH
Related information
- PubChem Compound (MeSH Keyword)
LinkOut - more resources
Full text sources.
- Ovid Technologies, Inc.
- Silverchair Information Systems
- Genetic Alliance
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Open access
- Published: 02 July 2024
Increased high-density lipoprotein cholesterol in patients with type 2 diabetes and its correlates: a cross-sectional, matched case–control survey
- Fatemeh Heydarzadeh 1 ,
- Fatemeh Mohammadi 1 ,
- Amirhossein Yadegar 1 ,
- Ali Mohammadi Naeini 1 ,
- Seyed Ali Nabipoorashrafi 1 ,
- Soghra Rabizadeh 1 ,
- Alireza Esteghamati 1 &
- Manouchehr Nakhjavani 1
European Journal of Medical Research volume 29 , Article number: 355 ( 2024 ) Cite this article
150 Accesses
Metrics details
So far, high-density lipoprotein cholesterol (HDL-C) levels and mortality were shown to have a U-shaped relationship. Additionally, high HDL-C levels increase the risk of developing a variety of diseases. However, a paucity of data exists regarding the characteristics of people with high HDL-C levels. The aim of this study was to assess the demographics and characteristics of patients with high HDL-C levels and compare their features with normal and low HDL-C groups.
As a cross-sectional, matched case–control study, a total of 510 patients with type 2 diabetes (T2D) were enrolled in the study and categorized into three matched groups according to their HDL-C concentrations. The studied groups were matched by their age and gender. Restricted cubic spline (RCS) curves were designed to evaluate the relationship between height, blood pressure, triglyceride, and vitamin D concentrations with the probability of having high HDL-C levels. Furthermore, violin plots were conducted to illustrate the distribution of continuous variables within each group.
This study showed that having high HDL-C (more than 70 mg/dL) compared to having low HDL-C (less than 40 mg/dL in men and 50 mg/dL in women) was significantly associated with height (OR 0.918, 95% CI 0.866–0.974), systolic blood pressure (SBP) (0.941, 0.910–0.972), vitamin D (0.970, 0.941–0.999), and triglyceride (0.992, 0.987–0.998) serum concentrations. Further analysis investigated that having high HDL-C levels compared to desired HDL-C levels (40 ≤ HDL-C levels < 70 in men and 50 ≤ HDL-C levels < 70 in women) was inversely associated with having SPB values greater than 130 mmHg. Besides, sufficient vitamin D levels (above 20 ng/ml) could 0.349 times decrease the odds of having high HDL-C versus normal HDL-C levels.
Sufficient vitamin D levels, SPB values higher than 130 mmHg, as well as increased triglyceride levels, were inversely associated with having high HDL levels. However, higher height values were associated with a decreased likelihood of having high HDL.
Introduction
High-density lipoprotein cholesterol (HDL-C) is a small lipoprotein with a complex structure determined by various particle sizes [ 1 ]. The prevalence of reduced HDL-C levels in the general population, less than 40 in men and 50 in women, has been reported to be 41.1% in Africa, followed by 35.4% in Europe, 33.4% in Korea, 21.1% in Turkey, 19.2% in China, 12.4%-33.1% in the United States, and 42% in Iran [ 2 , 3 , 4 ]. Literature has confirmed that low concentrations of HDL-C are associated with infectious diseases, sepsis-related death, diabetes, chronic kidney disease, various autoimmune diseases, and ruptured intracranial aneurysms [ 5 , 6 ].
Furthermore, the prevalence of high HDL-C levels greater than 70, was 26.6% among selected women in Tunisia [ 7 ]. High HDL-C levels were also reported in more than a third of type 1 diabetes (T1D) cases [ 8 ]. Potential regulatory roles are pictured for HDL-C [ 1 , 9 ]. HDL-C can directly regulate glucose metabolism and results in its antidiabetic effects. Current evidence has suggested that high HDL-C concentrations are risk factors for infectious diseases, pterygium, end-stage renal disease (ESRD) in lupus nephritis, increased insulin resistance in metabolic syndrome, and all-cause mortality [ 5 , 10 , 11 , 12 , 13 ]. Accordingly, a U-shaped relationship between HDL-C levels and cardiovascular and cancer mortality has been plotted [ 14 ].
Recent evidence supports the idea that high HDL-C levels (>50 mg/dL) can increase the cancer mortality rate [ 7 ]. The antioxidant effects of HDL-C have been manifested in prostate, lung, and endometrial cancer [ 15 ]. However, breast cancer could be promoted by HDL-C through increased migration of cancer cells. A positive correlation between high HDL-C and cancer progression was also demonstrated regarding the role of scavenger receptor BI proteins (SRB-1) in facilitating HDL-mediated cholesterol ester absorption by tumor cells [ 16 ]. Low HDL-C levels were also suggested to be endangered for cancer-related death [ 17 ].
Earlier, an inverse linear association was described between HDL-C levels, cardiovascular events, and all-cause mortality. Atherosclerosis development could be protected through different HDL-C capacities, including cholesterol removal from the artery wall, vasodilation in endothelial cells, protective effects on low-density lipoprotein (LDL) oxidation, and anti-inflammatory effects[ 9 , 18 ]. To date, there is a lack of documented conclusive evidence for the effectiveness of HDL-C-raising drugs [ 9 , 19 , 20 ]. Although it has been shown that niacin and cholesterol ester transfer protein (CETP) inhibitors could lead to an increase in HDL-C levels, the cardiovascular risk was not substantially reduced using such medications [ 5 ]. However, the cardioprotective role of high HDL-C has been questioned recently[ 14 ]. Recently, the Multi-Ethnic Study of Atherosclerosis (MESA) hypothesized that there may be a link between high HDL-C levels (>60 mg/dL), and a greater incidence of myocardial scars and that high HDL-C levels are associated with greater interstitial fibrosis, which manifests as longer myocardial native T1 times and greater extracellular volume [ 21 ]. Chronic kidney disease (CKD), diabetes, and coronary artery disease can cause dysfunctional HDL-C production, altering its anti-inflammatory properties [ 22 ].
So far, several pieces of research have been conducted on the effects of low HDL levels on cancer, cardiovascular events, and diabetes [ 16 , 23 , 24 , 25 ]. Recent studies have reported failure in reducing cardiovascular events, insulin resistance, diabetic retinopathy, cancer mortality rate, and unexpectedly increasing mortality in subjects with elevated HDL‐C levels [ 7 , 13 , 14 , 26 ]. However, there is a paucity of data on the characteristics of those with high HDL-C levels. On the other hand, there is no definition of the maximum level of normal HDL-C so far. The present survey tried to shed light on other correlates of high HDL-C. Studying the demographic and laboratory findings of individuals with high HDL-C levels could further increase the insights of the scientists toward the elements that could be effective in low or high HDL management. This study tried to examine all the demographics and characteristics of patients with low, normal, and high HDL-C levels in three age- and gender-matched groups.
Materials and methods
Study design and population.
A total of 7391 consecutive patients with type 2 diabetes (T2D) who were referred to a university hospital affiliated with the Tehran University between 2016 and 2021 were retrospectively recruited. Individuals who were unwilling to participate in the study; who were pregnant; who used aspirin, oral contraceptive drugs, or antioxidant and vitamin supplements; who were not receiving statins; who had several other chronic conditions (i.e., thyroid dysfunction, history of liver cirrhosis, CKD, or cancer); who had a history of smoking; or who were consuming alcohol were excluded from the study. As a result, 6127 patients were enrolled in the study. In the matching process, age and sex were determined as matching variables. Subjects with low, normal, and high HDL levels were matched for age and sex. According to the power of the study as 0.95 and ‘α’ as 0.05, utilizing G*Power software version 3.1.9.2 (Universität Düsseldorf, Germany), a total number of 400 was calculated [ 27 ]. Therefore, in the study period, 510 participants with T2D were included. A total of 170 patients with low HDL-C, 170 patients with normal HDL-C, and 170 patients with high HDL-C were matched by sex and age.
A high ratio of the target population had middle socioeconomic status, most of whom were covered by insurance. Patients were taking oral antidiabetic drugs (OADs), insulin, or a combination of these drugs. Informed written consent was obtained from all study populations. This study was in accordance with the Declaration of Helsinki. The study received formal ethical approval from the local ethics committee of Tehran University of Medical Sciences.
Data collection
The baseline characteristics of the recruited participants, including general information (age, sex, body mass index (BMI), hypertension status, hyperlipidemia status, diabetes duration, height, weight, waist circumference, and hip circumference) and laboratory test results (LDL-C, HDL-C, triglyceride (TG), total cholesterol (TC), non-HDL-C, fasting blood glucose (FBG), two-hour postprandial glucose (2hPP), hemoglobin A1c (HbA1c), vitamin D, creatinine and urinary albumin) were recorded.
A portable stadiometer and calibrated balance beam scale were employed to determine height and weight, respectively. WC was assessed halfway between the lowest rib margin and the iliac crest. Body mass index (BMI, kg/m 2 ) was calculated by using weight (kg)/height 2 (m 2 ). BMI was classified into three categories: underweight (BMI<18.5 kg/m 2 ), normal (18.5 kg/m 2 ≤ BMI < 25 kg/m 2 ), and overweight/obese (BMI ≥ 25 kg/m 2 ). The cut-off values for waist circumference (WC) were considered 98 cm for males and 84 cm for females [ 28 , 29 ]. Blood pressure was recorded in the right arm in the sitting position after the participants had rested for 5 mins. The individual’s right arm was placed at heart level and blood pressure was measured with a calibrated mercury sphygmomanometer (Reishter, Germany). The recorded data included the average of the last two systolic and diastolic pressures. All measurements were accomplished with an accuracy of 0.1 cm. After 12 h of overnight fasting, blood samples were collected in tubes coated with ethylene diaminetetracetic acid. All the samples were kept on ice and centrifuged at 3000 rpm for 15 min at 4 °C. Cholesterol, HDL, LDL, and TG were measured using direct enzymatic colorimetry with a Technicon RA-analyzer (Pars Azmoon, Karaj, Iran). Fasting blood sugar (FBS) and two-hour postprandial glucose (2 hPP) were quantified via the glucose oxidase test. Glucose level was assessed by the glucose oxidase method with an intra-assay coefficient of variation (intra-assay CV = 2.1%; interassay CV = 2.6%). High-performance liquid chromatography (HPLC) (A1C, DS5 Pink kit; Drew, Marseille, France) was utilized to evaluate hemoglobin A1c (HbA1c) levels. Non-HDL-C was estimated by reducing HDL-C from total cholesterol. The following equation (log (TG/HDL-C)) was performed to compute the atherogenic index of plasma (AIP). (WC(cm)/(39,68+(1.88*BMI) *(TG/1.03) *(1.31/HDL) for men and (WC(cm)/(36,58+(BMI *1.89) *(TG/0.81) *(1.52/HDL) for women were assessed to calculate the visceral adiposity index (VAI). eGFR was measured by using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. Serum creatinine was measured using the Jaffe method (Pars Azmun, Karaj, Iran)
Urinary albumin concentrations were measured by immunoturbidimetry (Cecile Instruments, Cambridge, United Kingdom). The detection limit was established at 2 mg/L. Urinary albumin concentrations were evaluated by an immunoturbidimetric commercial kit (Randox, Antrim, UK) [ 30 , 31 , 32 ].
Definitions
The criteria for defining diabetes mellitus followed the guidelines of the American Diabetes Association (ADA) [ 33 ]. Dyslipidemia was described according to the NCEP ATP III (National Cholesterol Education Program-Adult Treatment Panel III) and AHA/ACC (The American Heart Association/The American College of Cardiology) guidelines [ 34 , 35 ]. Low HDL-C (< 40 mg/dL in men and < 50 mg/dL in women), high LDL-C (≥ 70 mg/dL), high total cholesterol (≥ 200 mg/dL), high non-HDL-C (≥ 130 mg/dL), high TG (≥ 150 mg/dL), and high AIP (> 0.24) were defined according to guidelines. Microalbuminuria was defined as the excretion of between 30 and 300 mg/day of urine albumin.
Statistical analysis
All the statistical analyses were carried out using R software (version 4.2.3, R Foundation for Statistical Computing, Vienna, Austria) and IBM SPSS software version 24.0 (SPSS Inc., Chicago, Illinois, USA). The normality of the data was assessed with the Shapiro–Wilk test. Normal distribution quantitative values were represented by the mean ± standard deviation (SD) such as age, whereas data with no normal distribution was displayed as the median [interquartile range (IQR)], including duration of diabetes (years), weight (kg), height (kg), BMI (kg/m 2 ), WC (cm), hip circumference (cm), SBP (mm Hg), DBP (mm Hg), serum levels of vitamin D, total cholesterol (mg/dL), LDL-C (mg/dL), HDL-C (mg/dL), TG (mg/dL), non-HDL-C (mg/dL), AIP, TG/HDL ratio, HbA1c (%), FBS (mg/dL), 2 hPP (mg/dL), creatinine (mg/dL), GFR (ml/min/1.73 m 2 ), and microalbuminuria (mg/12 h). IQR was defined as the range between the first and third quartiles. Categorical parameters were expressed in terms of percentages and numbers [ n (%)]. Of 6127 patients, 510 subjects were matched by age and gender and assigned to three groups according to their HDL-C levels. The first group consisted of patients with low HDL-C levels (HDL-C < 40 in men, HDL-C < 50 in women), the second group contained those with normal HDL-C levels (40 ≤ HDL-C < 70 in men, 50 ≤ HDL-C < 70 in women), and the third group had high HDL-C levels (70 ≤ HDL-C). Each group contained 170 age-and-gender-matched individuals. ANOVA and Kruskal-Wallis tests were applied along with their Post Hoc pairwise comparisons to evaluate the baseline features of patients with normal and non-normal distributions. Between-group comparisons were assessed using Chi-square. Binary conditional logistic regression was employed to assess odds ratios (OR) and their 95% confidence intervals (CI) of patient’s characteristics for having high HDL-C levels. Univariate and multivariate regression models were analyzed. Multivariate model 1 was adjusted for height, WC, SBP, Vitamin D, HBA1c, the status of hypertensive drugs, the anti-diabetic drugs, the type of lipid-lowering drug, duration of diabetes, creatinine and triglyceride concentration. Multivariate model 2 was adjusted for height, SBP, Vitamin D, the status of hypertensive drugs, the anti-diabetic drugs, the type of lipid-lowering drug, duration of diabetes, and triglyceride concentration after dividing into different categories. Restricted cubic spline (RCS) curves in Fig. 1 with 4 knots were further utilized to explore the relationships between height (Fig. 1 C), SBP (Fig. 1 A), triglyceride (Fig. 1 D), and vitamin D (Fig. 1 B) concentrations with the risk of having high HDL-C levels. Violin plots (Fig. 2 ) were also designed to reveal the distribution of continuous variables, which were significantly different among the three groups. A two-sided P-value of less than 0.05 was assumed to be the threshold for statistical significance.
Association between systolic blood pressure (SBP), Vitamin D, height, and triglyceride and high HDL-C levels. The RCS models were used to analyze the relationship between SBP ( A ), vitamin D ( B ), height ( C ) and triglyceride ( D ) concentrations, and the probability of having HDL-C levels of more than 70. Each RCS consisted of four knots based on the distribution of the associated variable. The reference values for the abovementioned curves were as follows: height of 160 cm, SBP of 130 mm Hg, triglyceride levels of 150 mg/dl, and vitamin D levels of 20 mg/dl. HDL-C: high-density lipoprotein cholesterol
Violin plots of height, systolic blood pressure (SBP), triglyceride, and vitamin D levels for increased HDL-C levels. Violin plots were used to visually represent the density of height ( A ), SBP ( B ), triglyceride ( C ), and vitamin D ( D ) levels groups with high (70 ≤ HDL-C), normal HDL-C levels (40 ≤ HDL-C < 70 in men, 50 ≤ HDL-C < 70 in women), and low HDL-C levels (HDL-C <40 in men, HDL-C <50 in women). The median height was 161.5 cm, 160 cm, and 158 cm in the low HDL-C, normal HDL-C, and high HDL-C groups, respectively (Fig. 2 A). The group with high HDL-C levels had significantly lower SBP with a median value of 125 mm Hg (Fig. 2 B). Individuals with HDL-C levels of more than 70 had significantly lower levels of triglyceride [122 (IQR, 89–126)] and vitamin D [22 (IQR, 15–30)]. *p-value < 0.05 vs group with normal HDL, **p-value < 0.05 vs group with low HDL, HDL-C: high-density lipoprotein cholesterol
Baseline characteristics
In this study, 510 patients with T2D who were matched by their age and gender were stratified into three strata based on their HDL-C levels. The mean (± SD) age of the total participants was 59.83 ± 9.48 years. A majority of the population consisted of women, approximately 76.7%. The median duration of diabetes was six years (minimum five and maximum 38 years). The median BMI (kg/m 2 ) was 27.99 (IQR, 25.00–31.23). About 42.4% of participants were receiving anti-hypertensive drugs. The median values of SBP and DBP (mmHg) were 130 and 80, with ranges of 85 to 190 and 50 to 103, respectively. Serum levels of vitamin D had a median of 25 (ng/ml)(IQR, 20.00–32.00). All individuals were receiving statins, including atorvastatin (81%) and rosuvastatin (19 %). The median concentrations of total cholesterol (mg/dL), LDL-C (mg/dL), and HDL-C (mg/dL) were 170 (IQR, 145.00–205.00), 90 (IQR, 68.00–115.00), and 53 (IQR, 42.00–72.00), respectively. The median TG level was 136 (IQR, 99.00–190.00). All participants were taking antidiabetic agents, including OAD (76.4%), insulin (14.7%), and their combination (8.7%). Despite diabetes medication use, the median of HbA1c was 7.50 (IQR, 6.50–8.80), followed by 145 (IQR, 120.00–187.00) and 199.5 (IQR, 150.00–256.50) for FBS (mg/dL) and 2hPP (mg/dL), respectively. Microalbuminuria (mg/12 h) was computed to have a median of 7.50, along with a range of 4 to 13.57, respectively. Retinopathy and neuropathy were diagnosed in about 6.1% and 12.2% of the studied population. A total of 12.5% of participants reported a history of cardiovascular disease (CVD) in their visits.
Detailed profiles of participants in three categories stratified by HDL-C levels (defined as low HDL-C levels (HDL-C < 40 in men, HDL-C < 50 in women), normal HDL-C levels (40 ≤ HDL-C < 70 in men, 50 ≤ HDL-C < 70 in women), and high HDL-C levels (70 ≤ HDL-C)) were also investigated. A notable discrepancy was recorded in the duration of diabetes, height, waist circumference, SBP, vitamin D levels, lipid profile and parameters, and microalbuminuria among the three groups. Weight and non-HDL-C levels were significantly different in the high HDL-C group versus the low HDL-C group. In addition, individuals in the high HDL-C category had substantially greater differences in BMI and hip circumference than did those in the normal HDL-C category (Table 1 ) All demographic, anthropometric, clinical, and laboratory details of individuals in each classification are presented in Table 1 .
To visualize the smoothed density of height, SBP, triglyceride, and vitamin D among patients with high(70 ≤ HDL-C), normal(40 ≤ HDL-C < 70 in men, 50 ≤ HDL-C < 70 in women), and low(HDL-C < 40 in men, HDL-C < 50 in women) HDL-C levels, violin plots were constructed. Violin plot results revealed that the median height was 161.5 cm, 160 cm, and 158 cm in the low HDL-C, normal HDL-C, and high HDL-C groups, respectively (Fig. 2 A). As shown in Fig. 2 B the median SBP was 125 mmHg and the group with high HDL-C levels had significantly lower SBP values. Plots for triglyceride (Fig. 2 C) and vitamin D (Fig. 2 D) concentrations followed a similar pattern, which demonstrated that those with HDL-C levels greater than 70 had considerably lower levels of triglyceride [122 (IQR, 89–126)] and vitamin D [22(IQR, 15–30)].
Associations of the studied values with high HDL-C concentrations and low HDL-C concentrations
According to the crude models of conditional logistic regression analysis, there was a substantial negative association between HDL-C levels and height, weight, SBP, vitamin D, and triglycerides. In addition, after categorizing the variables, which had a significant association with having high HDL levels, into two groups according to their reference points in the RCS, their OR remained significant (Table 2 ) No considerable associations were illustrated between having a normal HDL-C level and having other HDL-C levels.
After further adjustments in the multivariable model, having high HDL-C levels compared to having low HDL-C levels remained significantly associated with height (OR 0.918, 95% CI 0.866–0.974), SBP (0.941, 0.910–0.972), vitamin D (0.970, 0.941–0.999), and triglyceride (0.992, 0.987–0.998) serum concentrations. After controlling for other variables, patients with heights less than 160 cm, SBP below 130 mm Hg, Vitamin D levels of less than 30 ng/ml, and triglyceride concentrations of less than 150 mg/dL were more susceptible to having HDL-C levels greater than 70 than to having low HDL-C levels (Table 3 )
Associations of the studied values with high HDL-C concentrations and normal HDL-C concentrations
In the unadjusted analysis, substantial relationships were detected between high HDL-C and height (0.971, 0.948–0.995), waist circumference (0.9971, 0.948–0.995), SBP (0.974, 0.960–0.988), and Vitamin D level (0.969, 0.947–0.991) (Table 4 ). However, after adjustments for confounding variables, only height, SBP, and vitamin D concentrations remained significantly associated with having high HDL-C compared to having normal HDL-C levels. Further analysis revealed that a diagnosis of SPB greater than 130 mm Hg could be inversely associated with having HDL-C levels greater than 70 mm Hg compared to having the desired HDL-C values. In addition, sufficient vitamin D concentrations (above 20 ng/ml) could 0.349 times decrease the odds of having high HDL-C versus normal HDL-C levels (Table 5 ).
The RCS models illustrated that SBP below 130 mmHg (A), Vitamin D less than 20 mg/dL (B), height less than 160 cm (C), and triglyceride less than 150 mg/dL (D) concentrations were positively correlated with the probability of having HDL-C levels of more than 70 (Fig. 1 ). Each RCS had four knots based on the distribution of the associated variable. The reference values for the abovementioned curves were as follows: height of 160 cm, SBP of 130 mm Hg, triglyceride levels of 150 mg/dL, and vitamin D levels of 20 mg/dL.
The current cross-sectional, matched case–control study aimed to determine the correlates of high HDL-C levels. To date, many studies have been performed to evaluate the relationship between dyslipidemia and the risk of cardiovascular and diabetic complications; however, this study focused on individuals with high HDL levels and showed that having lower values of height, systolic blood pressure, triglycerides, and vitamin D increased the odds of having high HDL-C levels.
The current analysis demonstrated that there is no significant distinction in BMI and weight between individuals with high and low values of HDL-C as well as those with normal values of HDL-C. BMI and weight were greater in patients with both low and high HDL-C levels than in those with normal HDL-C levels. A cohort study in a Japanese population showed that women with a BMI ≥ 25.0 kg/m 2 had a 1.54-fold greater risk of having low HDL-C concentrations than women with a BMI < 25.0 kg/m 2 [ 36 ]. Ali, H.I., et al. reported that abdominal obesity and overweight were likely to increase the risk of having high total cholesterol, LDL-C, and TG and decrease HDL-C in adults [ 37 ]. Patients with high levels of HDL-C demonstrated a reduction in SBP as opposed to those with low and normal levels of HDL-C. K.-H. Cho et al. demonstrated that a decrease in HDL-C has the potential to increase SBP. This occurs as a result of increased binding of LDL to the scavenger receptor B-I (SR-BI) due to low levels of HDL-C. The SRB-I receptor in mitochondria produces aldosterone by signaling [ 38 ].
This analysis showed that individuals with high HDL-C (HDL-C levels of more than 70) had lower SBP. Actually, patients with SBP below 130 mm Hg had a lower risk of having high HDL-C levels than low and normal HDL-C levels. Based on the research conducted by Deng et al., elevated TC, LDL-C, and non-HDL-C can potentially lead to hypertension by elevating the levels of circulating endothelin-1. As a result, there was an inverse association between HDL-C and the incidence of hypertension [ 39 ]. Nakajima et al. reported an inverted J-shaped association between HDL-C and hypertension risk (≥ 140/90 mm Hg) in both sexes [ 40 ]. The present analysis also endorsed these findings. Furthermore, a different study discovered a correlation between higher DBP and SBP and lower levels of HDL-C [ 38 ]. HDL-C may contribute to a decreased angiotensin II response by reducing NAD(P)H oxidase activity and aortic angiotensin II type 1 receptor expression and also increasing endothelial NO synthase dimerization in contrast to LDL and oxidized LDL [ 41 ]. These findings were consistent with the current investigation that illustrated an inverse association between high HDL-C and blood pressure. Previous studies have shown that HDL-C was positively associated with hypertension due to the role of hypertension in the disturbance of HDL metabolism and the increased percentage of individuals with dysfunctional HDL-C. In hypertensive individuals, some HDL-C particles may not function to protect LDL from oxidation or control cholesterol efflux from the walls of blood vessels [ 42 , 43 ]. However, Yang et al. proposed that HDL-C was not associated with systolic BP or hypertension in men [ 44 ].
The present survey discovered that the likelihood of having high HDL-C levels decreases as waist circumference and height increase. In addition, this analysis showed that the risk of having high HDL-C was higher in patients with heights less than 160 cm than in patients with heights more than 160 cm. Weschenfelder et al. demonstrated that serum triglyceride levels and waist circumference were significantly associated with lower HDL-C levels and smaller HDL-C particles in patients with heart failure [ 28 ]. Rosenbaum et al. reported that increasing waist circumference is associated with lower levels of large HDL particles (HDL2) and higher levels of small HDL particles (HDL3). In diabetes and cardiovascular disease, HDL3 has reduced anti-oxidative activity in patients with metabolic syndromes [ 45 ]. Williams et al. suggested that after one year, the weight loss resulted in a significant increase in HDL cholesterol, HDL2 cholesterol, and HDL2 mass[ 46 ]. Schekatolina et al. implied that Chylomicrons and VLDL were lipoprotein classes, rich in triglycerides, also known as TG-rich lipoproteins (TGRL). The metabolism of lipoprotein classes in the bloodstream is regulated through various pathways. The metabolism of HDL is specifically related to that of TGRL through the exchange of core lipids facilitated by the cholesteryl ester transfer protein (CETP), as well as through the transfer of surface fragments of TGRL to HDL that are generated during lipolysis by lipoprotein lipase (LPL). As a result, they showed a negative correlation between the concentrations of HDL-C and TG in circulation [ 47 ]. Miller and colleagues also demonstrated that a reduction of 50 mg/dl in triglyceride (TG) levels was associated with a 0.5 mg/dl increase in HDL-C levels in patients with TG levels of 200 mg/dl or higher. In addition, they found that the same reduction of TG levels resulted in a 1.7 mg/dl increase in HDL-C levels in individuals with TG levels below 200 mg/dl [ 48 ]. Another study showed that raising the plasma concentration of TG resulted in a greater decrease in HDL-C levels than in HDL-apolipoprotein A-I levels [ 49 ].
Navti et al. also suggested that increased BMI, waist circumference, and waist-to-height ratio were associated with lower HDL levels by increasing HDL-C catabolism due to insulin resistance, which suppresses lipolysis in an urban pediatric population in Cameroon [ 50 ]. Based on the current results, taller individuals exhibited decreased levels of HDL-C. Consistent with these results, Oh et al. implied that a decrease in sex hormones, an increase in insulin resistance, and damage to lecithin cholesterol acyl transferase activity could lead to an inverse relationship between height and HDL-C among patients with metabolic syndrome which could be attributed to the impact of aging on the metabolism of HDL [ 51 ]. A. M. Dattilo demonstrated that Subjects at a stabilized, reduced weight experienced a 0.009-mmol/L increase (P ≤ 0.01) in HDL-C per kilogram decrease in body weight. [ 52 ]. On the other hand, Shimizu et al. showed a significant positive correlation between height and HDL-C concentrations in individuals with BMI values greater than 25 kg/m 2 [ 53 ]. A study also suggested that HDL-C was positively correlated with overweight in Korean adolescents and adults [ 54 ]. Freedman, D. S. et al. reported positive associations of BMI with TG and inverse associations with HDL-C in black and white children [ 55 ]. Another study suggested that BMI had a negative influence on HDL-C activity by prohibiting platelet accumulation and cholesterol efflux capacity and reducing large HDL-C subfractions. High HDL-C levels may enhance small HDL-C particles among the obese population [ 56 ].
A meta-analysis in 2021 demonstrated that vitamin D administration in postmenopausal women reduced HDL-C levels, especially when the HDL-C baseline value was more than 50 mg/dL in overweight women [ 57 ]. These findings align with the current results, which revealed that Vitamin D concentrations less than 30 ng/ml were associated with a decrease in the odds of having high HDL-C levels versus low HDL-C levels. Moreover, patients with vitamin D concentrations above 20 ng/ml were more likely to have HDL-C levels above 70 than those with normal HDL-C levels. Literature reported that 25(OH) vitamin D levels could improve lipid profiles. Furthermore, vitamin D was shown to have an inverse association with total cholesterol, low-density lipoprotein cholesterol, and triglycerides. However, how vitamin D impacts the lipid profile is not yet clear. Vitamin D may decrease cholesterol biosynthesis via increased 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA) activity [ 58 ]. K. F. Faridi et. al. reported a positive association between vitamin D and more cardioprotective HDL-C particles via the role of vitamin D in reverse cholesterol transport. Furthermore, reverse cholesterol transport brings cholesterol out of lipid-laden macrophage sponge cells in atherosclerotic, plaques, such as HDL-C, for clearance from the circulation. Vitamin D deficiency could be related to impaired B-cell function which leads to insulin resistance, disruption of lipoprotein metabolism, increased TG levels, and decreased HDL cholesterol levels. Previous data have recommended that raising intestinal calcium absorption could lower the synthesis and secretion of hepatic TG. Therefore, vitamin D could arouse intestinal calcium absorption and prohibit TG synthesis and secretion. It has also been suggested that calcium could inhibit the intestinal absorption of fatty acids due to the formation of insoluble calcium–fatty acid complexes. Reduced absorption of fat, particularly saturated fatty acids, leads to reduced cholesterol concentrations in the serum. In addition, calcium could reduce the level of cholesterol by exciting the transformation of cholesterol into bile acids. Other studies have noted that vitamin D deficiency results in increased parathyroid hormone levels, which results in elevated TG and increased concentrations of vitamin D, decreasing serum PTH levels. This mechanism could influence TG concentrations [ 59 , 60 , 61 ]. Vitamin D supplementation could also decrease the HbA1c percentage by about 0.5% in T2DM patients. Therefore, a lack of vitamin D may cause dyslipidemia in elderly individuals with metabolic disorders [ 62 , 63 ]. Research has shown that there is no significant impact of vitamin D supplementation on HDL levels in prediabetic individuals [ 64 ]. The traditional Japanese diet consists of a high intake of fish, miso, soy sauce, and vegetables; contributes to the prevention of ASCVD; lowers the risk for hypercholesterolemia, CAD, and non-HDL-C and increases serum HDL-C levels by genetic deficiency of cholesterol ester transfer protein (CETP) [ 65 , 66 ].
These findings confirm the need for further investigation into the complexity of HDL to address impairments in HDL levels.
Limitations
This study should be interpreted in light of its potential limitations. As this study is cross-sectional, it is not possible to determine the clinical impact of these results. Additionally, it is difficult to discern whether the relation between HDL-C and these variants is a true effect, a survivor effect, or a cohort effect. Therefore, it would be valuable to conduct cohorts to track the HDL-C levels in each patient with different variables. The study population was limited to individuals with T2D, which hinders the generalizability of the results. Second, potential confounding factors not obtained in the present study might have affected the findings. Since the study population was limited to the capital city, it is not possible to apply the results to a wider population. Hence, prospective longitudinal studies with larger sample sizes are recommended. Finally, the average age of about 60 years in the studied population and the coverage of the majority of the individuals by women could also limit the results of the study.
The key strength of the current survey was that three groups of HDL-C levels were matched by age and gender in this analysis. Considering the low frequency of high HDL-C in the population, a total of 170 patients with type 2 diabetes and HDL-C levels of more than 70 mg/dL were included in the current study. Until now, most research has focused on low levels of HDL and its complications; however, this study described the importance of focusing on high HDL levels.
Due to recent findings that challenge the paradigm that high HDL-C levels are cardioprotective and because of a pattern of a U-shaped distribution of HDL-C, both low and high levels of HDL-C could be harmful. Therefore, strict control of the serum level of HDL-C may help in reducing the risk of cardiovascular events. This study focused on high HDL levels and the findings of the present study showed that SBP below 130 mm Hg, height less than 160 cm, Vitamin D less than 30 ng/ml, and lower triglyceride levels were positively associated with an increased likelihood of having high HDL-C levels. Further studies of high HDL-C levels are warranted to identify the causal role of HDL in health and disease states.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
High-density lipoprotein cholesterol
Type 1 diabetes
End-stage renal disease
Scavenger receptor BI proteins
Low-density lipoprotein
Cholesteryl ester transfer protein
Chronic kidney disease
Oral antidiabetic drugs
Body mass index
- Triglyceride
Total cholesterol
Fasting blood glucose
Two-hour postprandial glucose
Hemoglobin A1c
Waist circumference
Fasting blood sugar
High-performance liquid chromatography
Atherogenic index of plasma
Visceral adiposity index
Chronic Kidney Disease Epidemiology Collaboration
American Diabetes Association
National Cholesterol Education Program-Adult Treatment Panel III
The American Heart Association
The American College of Cardiology
Confidence interval
Restricted cubic spline
3-Hydroxy-3-methylglutaryl-coenzyme A reductase
Sirtori CR, Corsini A, Ruscica M. The role of high-density lipoprotein cholesterol in 2022. Curr Atheroscler Rep. 2022;24(5):365–77.
Article CAS PubMed PubMed Central Google Scholar
Toori MA, et al. Prevalence of hypercholesterolemia, high LDL, and low HDL in Iran: a systematic review and meta-analysis. Iran J Med Sci. 2018;43(5):449.
Google Scholar
Noubiap JJ, et al. Prevalence of dyslipidaemia among adults in Africa: a systematic review and meta-analysis. Lancet Glob Health. 2018;6(9):e998–1007.
Article PubMed Google Scholar
Bruckert E, et al. High prevalence of low HDL-cholesterol in a pan-European survey of 8545 dyslipidaemic patients. Curr Med Res Opin. 2005;21(12):1927–34.
Article CAS PubMed Google Scholar
von Eckardstein A, et al. High-density lipoprotein revisited: Biological functions and clinical relevance. Eur Heart J. 2022. https://doi.org/10.1093/eurheartj/ehac605 .
Article Google Scholar
Yadegar A, et al. Correlation between different levels and patterns of dyslipidemia and glomerular filtration rate in patients with type 2 diabetes: a cross-sectional survey of a regional cohort. J Clin Lab Anal. 2023;37(13–14): e24954.
Cherifa FB, et al. Prevalence of high HDL-cholesterol and its associated factors among childbearing age Tunisian Women: a cross-sectional study. Res Sq. 2021. https://doi.org/10.21203/rs.3.rs-154077/v1 .
Alessa T, et al. High HDL-C prevalence is common in type 1 diabetes and increases with age but is lower in Hispanic individuals. J Diabetes Complicat. 2015;29(1):105–7.
Mohammadi F, et al. Correlates of normal and decreased HDL cholesterol levels in type 2 diabetes: a cohort-based cross-sectional study. Lipids Health Dis. 2024;23(1):18.
Zang S, et al. High HDL-C and high LDL-C are risk factors of pterygium in a population-based cross-sectional study in Southern China: the Dongguan Eye Study. BMJ Open. 2022;12(6): e058649.
Article PubMed PubMed Central Google Scholar
Can A, et al. Lipid-lowering agents and high HDL (high-density lipoprotein) are inversely associated with intracranial aneurysm rupture. Stroke. 2018;49(5):1148–54.
Yin P, et al. Effect of low and high HDL-C levels on the prognosis of lupus nephritis patients: a prospective cohort study. Lipids Health Dis. 2017;16(1):1–9.
Hiratsuka N, et al. Significance of high HDL cholesterol levels in Japanese men with metabolic syndrome. Intern Med. 2011;50(19):2113–20.
Yan YQ, Chen J, Huang YQ. A Non-linear association of high-density lipoprotein cholesterol with all-cause and cause-specific mortality in diabetic patients. Diabetes Metab Syndr Obes Targets Ther. 2021. https://doi.org/10.2147/DMSO.S313006 .
Yadegar A, et al. Prevalence of different patterns of dyslipidemia in patients with type 2 diabetes in an Iranian population. Transl Med Commun. 2022;7(1):23.
Patel KK, Kashfi K. Lipoproteins and cancer: the role of HDL-C, LDL-C, and cholesterol-lowering drugs. Biochem Pharmacol. 2022;196: 114654.
Ben Cherifa F, et al. Prevalence of high HDL cholesterol and its associated factors among Tunisian women of childbearing age: a cross-sectional study. Int J Environ Res Public Health. 2021;18(10):5461.
van der Vorst EP. High-density lipoproteins and apolipoprotein A1. In: Hoeger U, Harris JR, editors. Vertebrate and invertebrate respiratory proteins, lipoproteins and other body fluid proteins. Cham: Springer International Publishing; 2020. p. 399–420.
Chapter Google Scholar
Olsson AG. Is high HDL cholesterol always good? Ann Med. 2009;41(1):11–8.
Yadegar A, et al. Decreasing trend of blood lipid profile in type 2 diabetes: not a promising change in HDL-C, a serial cross-sectional study. PLoS ONE. 2023;18(10): e0293410.
Chehab O, et al. Higher HDL cholesterol levels are associated with increased markers of interstitial myocardial fibrosis in the MultiEthnic Study of Atherosclerosis (MESA). Sci Rep. 2023;13(1):20115.
Zewinger S, et al. Symmetric dimethylarginine, high-density lipoproteins and cardiovascular disease. Eur Heart J. 2017;38(20):1597–607.
Chapman MJ. HDL functionality in type 1 and type 2 diabetes: new insights. Curr Opin Endocrinol Diabetes Obes. 2022;29(2):112.
Yang HS, et al. Sex-specific U-shaped relationships between high-density lipoprotein cholesterol levels and 10-year major adverse cardiovascular events: a nationwide cohort study of 5.7 million South Koreans. Ann Lab Med. 2002;42(4):415–27.
Qahremani R, et al. Lipid profile, ox-LDL, and LCAT activity in patients with endometrial carcinoma and type 2 diabetes: the effect of concurrent disease based on a case–control study. Health Sci Rep. 2023;6(9): e1537.
Zhang C, et al. Association between high-density lipoprotein cholesterol to apolipoprotein A ratio and diabetic retinopathy: a cross-sectional study. J Diabetes Compl. 2023;37(6): 108471.
Article CAS Google Scholar
Faul F, et al. Statistical power analyses using G* Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–60.
Weir CB, Jan A. BMI classification percentile and cut off points. Treasure Island: StatPearls Publishing; 2019.
Tutunchi H, et al. What are the optimal cut-off points of anthropometric indices for prediction of overweight and obesity? Predictive validity of waist circumference, waist-to-hip and waist-to-height ratios. Health Prom Perspect. 2020;10(2):142.
Organization WH. Waist circumference and waist-hip ratio: report of a WHO expert consultation. Geneva. 2008;8–11:2011.
Amato MC, et al. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920–2.
Eknoyan G, et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney int. 2013;3(1):5–14.
AD Association. Erratum. Classification and diagnosis of diabetes Sec 2 In Standards of Medical Care in Diabetes-2016. Diabetes Care 2016; 39 (Suppl. 1): S13-S22. Diabetes Care. 2016;39(9):1653.
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). J Am Med Assoc. 2002. https://doi.org/10.1001/jama.285.19.2486 .
Grundy S, Stone N, Bailey A. A Repor of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73(24):e285-350.
Ohta T, et al. Relationship of cardiorespiratory fitness and body mass index with the incidence of dyslipidemia among Japanese women: a cohort study. Int J Environ Res Public Health. 2019;16(23):4647.
Ali HI, et al. Associations of dyslipidemia with dietary intakes, body weight status and sociodemographic factors among adults in The United Arab Emirates. Nutrients. 2022;14(16):3405.
Cho K-H, Park H-J, Kim J-R. Decrease in serum HDL-C level is associated with elevation of blood pressure: correlation analysis from the Korean National Health and nutrition examination survey 2017. Int J Environ Res Public Health. 2020;17(3):1101.
Deng G, Li Y, Cheng W. Association of lipid levels with the prevalence of hypertension in Chinese women: a cross-sectional study based on 32 health check centers. Front Endocrinol. 2022;13: 904237.
Nakajima K, et al. Association of serum high-density lipoprotein cholesterol with high blood pressures at checkup: results of Kanagawa investigation of total checkup data from the national database-9 (KITCHEN-9). J Clin Med. 2021;10(21):5118.
Van Linthout S, et al. Vascular-protective effects of high-density lipoprotein include the downregulation of the angiotensin II type 1 receptor. Hypertension. 2009;53(4):682–7.
Oda E, Kawai R. High-density lipoprotein cholesterol is positively associated with hypertension in apparently healthy Japanese men and women. Br J Biomed Sci. 2011;68(1):29–33.
Kawamoto R, et al. Increased high-density lipoprotein cholesterol is associated with a high prevalence of pre-hypertension and hypertension in community-dwelling persons. Endocrine. 2012;42:321–8.
Yang G, et al. Adjustment for body mass index changes inverse associations of HDL-cholesterol with blood pressure and hypertension to positive associations. J Hum Hypertens. 2022;36(6):570–9.
Rosenbaum D, et al. Waist circumference is a strong and independent determinant of the distribution of HDL subfractions in overweight patients with cardiovascular risk factors. Diab Vasc Dis Res. 2012;9(2):153–9.
Williams PT, et al. The effects of weight loss by exercise or by dieting on plasma high-density lipoprotein (HDL) levels in men with low, intermediate, and normal-to-high HDL at baseline. Metabolism. 1994;43(7):917–24.
Schekatolina S, et al. Mathematical modelling of material transfer to high-density lipoprotein (HDL) upon triglyceride lipolysis by lipoprotein lipase: relevance to cardioprotective role of HDL. Metabolites. 2022;12(7):623.
Miller M, Langenberg P, Havas S. Impact of lowering triglycerides on raising HDL-C in hypertriglyceridemic and non-hypertriglyceridemic subjects. Int J Cardiol. 2007;119(2):192–5.
Tremblay AJ, et al. Differential impact of plasma triglycerides on HDL-cholesterol and HDL-apo AI in a large cohort. Clin Biochem. 2007;40(1–2):25–9.
Navti LK, et al. An assessment of predisposing factors of atherogenic dyslipidemia in an urban pediatric population in cameroon. J Biosci Med. 2022;10(7):1–18.
CAS Google Scholar
Oh N-K, et al. Short stature is associated with increased risk of dyslipidemia in Korean adolescents and adults. Sci Rep. 2019;9(1):14090.
Dattilo AM, Kris-Etherton P. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56(2):320–8.
Shimizu Y, et al. Height correlates with dyslipidemia in non-overweight middle-aged Japanese men. J Physiol Anthropol. 2016;35(1):1–6.
Haile K, Haile A, Timerga A. Predictors of lipid profile abnormalities among patients with metabolic syndrome in Southwest Ethiopia: a cross-sectional study. Vasc Health Risk Manag. 2021. https://doi.org/10.2147/VHRM.S319161 .
Freedman DS, et al. Differences in the relation of obesity to serum triacylglycerol and VLDL subclass concentrations between black and white children: the Bogalusa Heart Study. Am J Clin Nutr. 2002;75(5):827–33.
Rezaee M, et al. BMI modifies HDL-C effects on coronary artery bypass grafting outcomes. Lipids Health Dis. 2022;21(1):128.
Liu W, et al. Vitamin D and lipid profiles in postmenopausal women: a meta-analysis and systematic review of randomized controlled trials. Front Mol Biosci. 2021;8: 799934.
Dibaba DT. Effect of vitamin D supplementation on serum lipid profiles: a systematic review and meta-analysis. Nutr Rev. 2019;77(12):890–902.
Faridi KF, et al. Serum vitamin D and change in lipid levels over 5 y: The Atherosclerosis Risk in Communities study. Nutrition. 2017;38:85–93.
AlQuaiz AM, et al. Association between standardized vitamin 25 (OH) D and dyslipidemia: a community-based study in Riyadh, Saudi Arabia. Environ Health Prev Med. 2020;25:1–9.
Filgueiras MDS, et al. Lower vitamin D intake is associated with low HDL cholesterol and vitamin D insufficiency/deficiency in Brazilian children. Public Health Nutr. 2018;21(11):2004–12.
Surdu AM, et al. Vitamin D and its role in the lipid metabolism and the development of atherosclerosis. Biomedicines. 2021;9(2):172.
Wenclewska S, et al. Vitamin D supplementation reduces both oxidative DNA damage and insulin resistance in the elderly with metabolic disorders. Int J Mol Sci. 2019;20(12):2891.
Yang Y, et al. Effects of vitamin D supplementation on the regulation of blood lipid levels in prediabetic subjects: a meta-analysis. Front Nutr. 2023;10:242.
Htun NC, et al. Dietary pattern and its association with blood pressure and blood lipid profiles among Japanese adults in the 2012 Japan National Health and Nutrition Survey. Asia Pac J Clin Nutr. 2018;27(5):1048–61.
CAS PubMed Google Scholar
Tani S, et al. Gender differences in the associations among fish intake, lifestyle, and non-HDL-C level in Japanese subjects over the age of 50 years: anti-atherosclerotic effect of fish consumption. Nutr Metab Cardiovasc Dis. 2021;31(5):1434–44.
Download references
Acknowledgements
The authors thank the patients and health staff for their worthwhile contributions.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Endocrinology and Metabolism Research Center (EMRC), Vali-Asr Hospital, Tehran University of Medical Sciences, Tehran, Iran
Fatemeh Heydarzadeh, Fatemeh Mohammadi, Amirhossein Yadegar, Ali Mohammadi Naeini, Seyed Ali Nabipoorashrafi, Soghra Rabizadeh, Alireza Esteghamati & Manouchehr Nakhjavani
You can also search for this author in PubMed Google Scholar
Contributions
Fatemeh Heydarzadeh: Conceptualization, Investigation, Writing – original draft, Data curation, Writing – review & editing; Fatemeh Mohammadi: Formal analysis, Methodology, Software, Visualization, Writing – review & editing; Amirhossein Yadegar: Formal analysis, Methodology, Software, Visualization, Writing – review & editing; Ali Mohammadi Naeini: Methodology, Formal analysis, Visualization, Investigation, Writing – original draft; Seyed Ali Nabipoorashrafi: Data curation, Writing – original draft; Soghra Rabizadeh; Methodology, Validation, Visualization; Alireza Esteghamati: Conceptualization, Visualization, Methodology, Supervision; Manouchehr Nakhjavani: Conceptualization, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing-Lead. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Manouchehr Nakhjavani .
Ethics declarations
Ethical approval and consent to participate.
The study received formal ethical approval from the local ethics committee of Tehran University of Medical Sciences.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Heydarzadeh, F., Mohammadi, F., Yadegar, A. et al. Increased high-density lipoprotein cholesterol in patients with type 2 diabetes and its correlates: a cross-sectional, matched case–control survey. Eur J Med Res 29 , 355 (2024). https://doi.org/10.1186/s40001-024-01950-0
Download citation
Received : 18 March 2024
Accepted : 25 June 2024
Published : 02 July 2024
DOI : https://doi.org/10.1186/s40001-024-01950-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cholesterol
- Lipoproteins
- High-density lipoprotein
European Journal of Medical Research
ISSN: 2047-783X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Consensus Guidance for Monitoring Individuals With Islet Autoantibody–Positive Pre-Stage 3 Type 1 Diabetes
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Moshe Phillip , Peter Achenbach , Ananta Addala , Anastasia Albanese-O’Neill , Tadej Battelino , Kirstine J. Bell , Rachel E.J. Besser , Ezio Bonifacio , Helen M. Colhoun , Jennifer J. Couper , Maria E. Craig , Thomas Danne , Carine de Beaufort , Klemen Dovc , Kimberly A. Driscoll , Sanjoy Dutta , Osagie Ebekozien , Helena Elding Larsson , Daniel J. Feiten , Brigitte I. Frohnert , Robert A. Gabbay , Mary P. Gallagher , Carla J. Greenbaum , Kurt J. Griffin , William Hagopian , Michael J. Haller , Christel Hendrieckx , Emile Hendriks , Richard I.G. Holt , Lucille Hughes , Heba M. Ismail , Laura M. Jacobsen , Suzanne B. Johnson , Leslie E. Kolb , Olga Kordonouri , Karin Lange , Robert W. Lash , Åke Lernmark , Ingrid Libman , Markus Lundgren , David M. Maahs , M. Loredana Marcovecchio , Chantal Mathieu , Kellee M. Miller , Holly K. O’Donnell , Tal Oron , Shivajirao P. Patil , Rodica Pop-Busui , Marian J. Rewers , Stephen S. Rich , Desmond A. Schatz , Rifka Schulman-Rosenbaum , Kimber M. Simmons , Emily K. Sims , Jay S. Skyler , Laura B. Smith , Cate Speake , Andrea K. Steck , Nicholas P.B. Thomas , Ksenia N. Tonyushkina , Riitta Veijola , John M. Wentworth , Diane K. Wherrett , Jamie R. Wood , Anette-Gabriele Ziegler , Linda A. DiMeglio; Consensus Guidance for Monitoring Individuals With Islet Autoantibody–Positive Pre-Stage 3 Type 1 Diabetes. Diabetes Care 2024; dci240042. https://doi.org/10.2337/dci24-0042
Download citation file:
- Ris (Zotero)
- Reference Manager
Given the proven benefits of screening to reduce diabetic ketoacidosis (DKA) likelihood at the time of stage 3 type 1 diabetes diagnosis, and emerging availability of therapy to delay disease progression, type 1 diabetes screening programs are being increasingly emphasized. Once broadly implemented, screening initiatives will identify significant numbers of islet autoantibody–positive (IAb + ) children and adults who are at risk for (confirmed single IAb + ) or living with (multiple IAb + ) early-stage (stage 1 and stage 2) type 1 diabetes. These individuals will need monitoring for disease progression; much of this care will happen in nonspecialized settings. To inform this monitoring, JDRF, in conjunction with international experts and societies, developed consensus guidance. Broad advice from this guidance includes the following: 1 ) partnerships should be fostered between endocrinologists and primary care providers to care for people who are IAb + ; 2 ) when people who are IAb + are initially identified, there is a need for confirmation using a second sample; 3 ) single IAb + individuals are at lower risk of progression than multiple IAb + individuals; 4 ) individuals with early-stage type 1 diabetes should have periodic medical monitoring, including regular assessments of glucose levels, regular education about symptoms of diabetes and DKA, and psychosocial support; 5 ) interested people with stage 2 type 1 diabetes should be offered trial participation or approved therapies; and 6 ) all health professionals involved in monitoring and care of individuals with type 1 diabetes have a responsibility to provide education. The guidance also emphasizes significant unmet needs for further research on early-stage type 1 diabetes to increase the rigor of future recommendations and inform clinical care.
Graphical Abstract

This article contains supplementary material online at https://doi.org/10.2337/figshare.25800055 .
This consensus report was endorsed by the European Society for the Study of Diabetes (EASD), American Diabetes Association (ADA), American Association of Clinical Endocrinology (AACE), American College of Diabetology (ACD), Association of Diabetes Care & Education Specialists (ADCES), Australian Diabetes Society (ADS), the International Society for Pediatric and Adolescent Diabetes (ISPAD), Advanced Technologies & Treatments for Diabetes (ATTD), DiaUnion, the Endocrine Society, and JDRF International.
This article is being simultaneously published in Diabetes Care ( https://doi.org/10.2337/dci24-0042 ) and Diabetologia ( https://doi.org/10.1007/s00125-024-06205-5 ) by the ADA and the EASD.
A consensus report is a document on a particular topic that is authored by a technical expert panel under the auspices of ADA. The document does not reflect the official ADA position but rather represents the panel’s collective analysis, evaluation, and expert opinion. The primary objective of a consensus report is to provide clarity and insight on a medical or scientific matter related to diabetes for which the evidence is contradictory, emerging, or incomplete. The report also aims to highlight evidence gaps and to propose avenues for future research. Consensus reports undergo a formal review process, including external peer review and review by the ADA Professional Practice Committee and ADA scientific team for publication.
Email alerts
- Online ISSN 1935-5548
- Print ISSN 0149-5992
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Diabetes patients on GLP-1s instead of insulin have lower cancer risk, study shows
By maggie fick, reuters | posted - july 5, 2024 at 5:39 p.m., patients with type 2 diabetes taking treatments like ozempic, have a lower chance of developing 10 types of obesity-related cancers than those taking other diabetes drugs, according to a study. (hollie adams, reuters).
Estimated read time: 2-3 minutes
LONDON — Patients with type 2 diabetes taking GLP-1 treatments, which include Ozempic, have a lower chance of developing 10 types of obesity-related cancers than those taking insulin and other diabetes drugs, according to a study published on Friday.
GLP-1 treatments for type 2 diabetes have been on the market for nearly 20 years. The newer generation — such as Novo Nordisk's Ozempic and Eli Lilly's Mounjaro — are far more effective at controlling blood sugar levels and inducing weight loss. Ozempic was the first of the newer generation in the class to be approved, in 2017.
In the study published on Friday in medical journal JAMA Network Open, researchers examined the medical records of 1.6 million patients with type 2 diabetes who had no prior history of 13 types of obesity-related cancers including gallbladder cancer and kidney cancer.
The study did not specify which GLP-1 medicines the patients took, but the records were for patients on these medicines or insulin or the diabetes drug metformin between March 2005 and November 2018. Ozempic was only approved by the U.S. Food and Drug Administration in December 2017.
The study found that the patients treated with a GLP-1 therapy instead of insulin "had a significant risk reduction" in 10 of those cancers.
The findings are "preliminary evidence of the potential benefit" of GLP-1 drugs for cancer prevention in high-risk population, the researchers concluded. They also said that studies of the newer generation of these medicines for their cancer preventative effects are warranted.
The authors of the study did not report having received funds from drugmakers who market these medicines.
The versions of these medicines that are approved to treat obesity, and have been shown to help patients lose as much as 20% of their weight on average, have exploded in popularity, leading to record profits for Novo and Lilly.
Lilly's Mounjaro and weight-loss therapy Zepbound, as well as Novo's rival medicines Ozempic and Wegovy are already being studied to see whether they can improve health in many other ways, ranging from alcohol addiction to sleep apnea.
In March, the U.S. Food and Drug Administration approved Wegovy for lowering the risk of stroke and heart attack in overweight or obese adults who do not have diabetes.
Related stories
Fda warns website over unlawful sale of weight-loss drugs, 'ozempic babies': reports of surprise pregnancies raise new questions about weight loss drugs, most recent health stories, early signs of dementia can show in your finances, a rare voice box transplant helped a cancer patient speak again in pioneering study, new alzheimer's drug wins approval after fda delay, related topics, more stories you may be interested in.

Youth group exposed to rabies during girl's camp in Idaho

These ultraprocessed foods may shorten your life, study says
Most viewed.
- Ogden business leader killed in weekend ATV crash
- 'It's a miracle': Cyclist lucky to keep arm following gruesome weekend crash in Provo
- Utah family argues discrimination at local waterpark
- Safety of Ogden Canyon Road focus of scrutiny in wake of deadly accident
- French court rules US man detained after 'So I raped you' Facebook message can be extradited
- Utah commission approves Enbridge plan to cut natural gas rate by nearly 30%
- Family believes pilot had medical emergency before deadly crash in Utah County
- Calls alleging blocked roads, harassment in Virgin lead to arrest of 2 convicted felons
- Rep. Phil Lyman requested the complete 2024 Utah voter rolls
- Lyman and Clawson sue for information on Utah voters who signed candidates' petitions
STAY IN THE KNOW

KSL Weather Forecast


IMAGES
VIDEO
COMMENTS
Type 1 diabetes (T1D) is a common autoimmune disease that is characterized by insufficient insulin production. ... This case-control study attempted to estimate the exposure linked to T1D to ...
Type 1 diabetes accounts for approximately 6% of all cases of diabetes in adults (≥18 years of age) ... observational case control studies. PLoS One 2013;8(6):e65326-e65326. ...
Risk factors for type 1 diabetes, including environmental, behavioural and gut microbial factors: a case-control study. ... The case-control study began in January 2016 46 and ended in September 2018 (case-control phase of clinicaltrial.gov Protocol ID: G12114000080001). The work was conducted following the STROBE Statement for a case ...
PRESENTATION OF CASE. Dr. Max C. Petersen (Medicine): A 34-year-old woman was evaluated in the diabetes clinic of this hospital for hyperglycemia. Eleven years before this presentation, the blood glucose level was 126 mg per deciliter (7.0 mmol per liter) on routine laboratory evaluation, which was performed as part of an annual well visit.
A recent case-control study showed that the incidence of vascular-wall inflammation was higher among patients with type 1 diabetes than among persons without diabetes who were matched for age ...
Hyperosmolar hyperglycemic state (HHS), a rare diabetic hyperglycemic emergency, is most often observed in adult patients, but seldom seen in pediatric patients. Nevertheless, it can present in younger adults and teenagers as the first presentation of diabetes mellitus type 2 (T2DM). 1
A 59-year-old woman with type 1 diabetes and a 2-year history of cognitive decline presented with obtundation. There was diffuse, symmetric hypointensity in the brain on T2-weighted images and abno...
Registered dietitians (RDs) who have earned the Board Certified-Advanced Diabetes Manager (BC-ADM) credential hold a master's or doctorate degree in a clinically relevant area and have at least 500 hours of recent experience helping with the clinical management of people with diabetes.1 They work in both inpatient and outpatient settings, including diabetes or endocrine-based specialty ...
Evidence suggests that genome-wide hypomethylation may promote genomic instability and cellular senescence, leading to chronic complications in people with diabetes mellitus. Limited data are however available on the Alu methylation status in patients with type 1 diabetes (T1D). Methods: We investigated DNA methylation levels and patterns of Alu methylation in the peripheral blood of 36 ...
Methods: A matched case-control study of Type 1 DM conducted in Lancashire and Cumbria, UK, using a structured interview. Cases (n=196, participation rate 83%) were children under 16 years of age diagnosed prior to October 1998 and attending diabetic clinics. Controls (n=381) were healthy children from the community matched by gender and by age ...
Material and methods: A case-control study was conducted on children £ 16 years old who were diagnosed with T1DM and healthy age and sex-matched controls. Data regarding the socio-demographic status, gestational and neonatal risk factors were evaluated. Results: One hundred and one children with T1DM (41 males and 60 females), and the same ...
Methods: A cross-sectional study was set-up to examine the metabolic profile in fasting plasma samples from seven children with poorly controlled T1DM and seven non-diabetic controls aged 8-18 years, and matched for gender, age and BMI-SDS. The obtained plasma 1 H-NMR spectra were rationally divided into 110 integration regions, representing ...
This study evaluated the body image perception in children with type 1 diabetes in order to identify symptoms of disordered eating behaviours early. Children with type 1 diabetes and controls showed underestimation and dissatisfaction with body size.
The relationship between infant breastfeeding and type 1 diabetes mellitus (DM) is unclear but it has been suggested that there may be a link between many environmental factors, including dietary antigens affecting diabetes epidemiology. The main objective of this study is to investigate nutritional risk factors, especially breastfeeding early in life that may be associated with the ...
This study was designed as a noninterventional cross-sectional case-control study. All subjects were enrolled at the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China. T1D patients were participants of the T1D China Registry Study ( www.chictr.org.cn , ChiCTR2000034642). 28 Inclusion criteria were participants diagnosed ...
Uncertainty still exists regarding the role of some environmental risk in the development of type 1 diabetes mellitus (T1DM) both globally and in Egypt. The objective here was to explore the potential environmental risk factors associated with the development of T1DM among children in Egypt. A case-controlled study of 204 T1DM children and an equal number of age and sex-matched controls was ...
The AUC was excellent (AUC = 0.996, P = 0.0001), with high diagnostic accuracy (88.2) in differentiating newly diagnosed type 1 diabetes mellitus from the healthy subject group. We demonstrated low irisin levels in type 1 diabetes mellitus and the association of the highest irisin amounts to an insulin therapy and a better glycaemic control.
This study used a data set from a prior case-control study to identify risk characteristics for SH in older adults with type 1 diabetes.4 The random forest algorithm was used to classify (ie, identify) cases versus controls based on individual-level characteristics (ie, covariates).
Results. A total of 104 patients with CHD and type 1 diabetes mellitus were matched with 520 controls. Patients with CHD and type 1 diabetes mellitus had an earlier onset of diabetes (13.9 versus 17.4 years, p<0.001), longer duration of diabetes (22.4 versus 18.1 years, p<0.001), higher prevalence of retinopathy (64.0 versus 43.0%, p=0.003), higher creatinine levels (83.5 versus 74.1 µmol/L ...
A case-control study was carried out with 16 children with type 1 diabetes and 16 healthy children. The fecal bacteria composition was investigated by polymerase chain reaction-denaturing gradient gel electrophoresis and real-time quantitative polymerase chain reaction. ... This is the first study showing that type 1 diabetes is associated with ...
This paper applies the CEG framework to a Yorkshire, United Kingdom, case-control study of childhood type 1 diabetes (1993-1994) in order to examine 4 exposure variables associated with the mother, 3 of which are fully observed (her school-leaving-age, amniocenteses during pregnancy, and delivery type) and 1 with missing values (her rhesus factor), while incorporating previous type 1 Diabetes ...
To the Editor: Most patients with new-onset type 1 diabetes have substantial intact beta-cell reserve. 1 Thus, we analyzed the efficacy of semaglutide, an agonist of glucagon-like peptide 1 (GLP-1 ...
Conclusions Machine learning may augment risk stratification for OAs by identifying key characteristics associated with SH. Prospective studies are needed to identify the predictive performance of these risk characteristics. Date of publication. 2024; Keyword. Blood Glucose; Diabetes Mellitus, Type 1; Case-Control Studies; Humans; Diabetes ...
Humanitarian health actors are beginning to better consider and manage non-communicable diseases, such as diabetes, in emergency and protracted crisis settings. However, a focus on the more globally prevalent type 2 diabetes (T2D) dominates. Blind spots prevail in the unmet needs for type 1 diabetes (T1D), a chronic autoimmune condition where individuals are unable to produce insulin, thereby ...
Background: Type 1 diabetes mellitus (T1DM) is the most common chronic autoimmune disease among children and adolescents. Telemedicine has been widely used in the field of chronic disease management and can benefit patients with T1DM. However, existing studies lack high-level evidence related to the effectiveness of telemedicine for glycemic control in children and adolescents with T1DM.
Data released by the Centers for Disease Control and Prevention earlier this year shows the U.S. is projected to have a significant increase in children diagnosed with diabetes.. According to the CDC, between now and 2060, the number of children with Type 2 diabetes is projected to increase by 700%, while cases of youths with Type 1 diabetes are expected to increase by 65%.
Study objectives: To assess differences in habitual sleep patterns and sleep states between children and adolescents with type 1 diabetes mellitus (T1DM) and control subjects, and to explore the relationships between sleep, glucose levels, and glycemic control. Methods: Participants included 82 children (5-18 years); 41 with T1DM (cases), and 41 healthy control subjects group matched for age ...
As a cross-sectional, matched case-control study, a total of 510 patients with type 2 diabetes (T2D) were enrolled in the study and categorized into three matched groups according to their HDL-C concentrations. ... Alessa T, et al. High HDL-C prevalence is common in type 1 diabetes and increases with age but is lower in Hispanic individuals ...
Once broadly implemented, screening initiatives will identify significant numbers of islet autoantibody-positive (IAb +) children and adults who are at risk for (confirmed single IAb +) or living with (multiple IAb +) early-stage (stage 1 and stage 2) type 1 diabetes. These individuals will need monitoring for disease progression; much of ...
In the study published on Friday in medical journal JAMA Network Open, researchers examined the medical records of 1.6 million patients with type 2 diabetes who had no prior history of 13 types of ...