- Open access
- Published: 24 April 2024

Breast cancer screening motivation and behaviours of women aged over 75 years: a scoping review
- Virginia Dickson-Swift 1 ,
- Joanne Adams 1 ,
- Evelien Spelten 1 ,
- Irene Blackberry 2 ,
- Carlene Wilson 3 , 4 , 5 &
- Eva Yuen 3 , 6 , 7 , 8
BMC Women's Health volume 24 , Article number: 256 ( 2024 ) Cite this article
922 Accesses
Metrics details
This scoping review aimed to identify and present the evidence describing key motivations for breast cancer screening among women aged ≥ 75 years. Few of the internationally available guidelines recommend continued biennial screening for this age group. Some suggest ongoing screening is unnecessary or should be determined on individual health status and life expectancy. Recent research has shown that despite recommendations regarding screening, older women continue to hold positive attitudes to breast screening and participate when the opportunity is available.
All original research articles that address motivation, intention and/or participation in screening for breast cancer among women aged ≥ 75 years were considered for inclusion. These included articles reporting on women who use public and private breast cancer screening services and those who do not use screening services (i.e., non-screeners).
The Joanna Briggs Institute (JBI) methodology for scoping reviews was used to guide this review. A comprehensive search strategy was developed with the assistance of a specialist librarian to access selected databases including: the Cumulative Index to Nursing and Allied Health Literature (CINAHL), Medline, Web of Science and PsychInfo. The review was restricted to original research studies published since 2009, available in English and focusing on high-income countries (as defined by the World Bank). Title and abstract screening, followed by an assessment of full-text studies against the inclusion criteria was completed by at least two reviewers. Data relating to key motivations, screening intention and behaviour were extracted, and a thematic analysis of study findings undertaken.
A total of fourteen (14) studies were included in the review. Thematic analysis resulted in identification of three themes from included studies highlighting that decisions about screening were influenced by: knowledge of the benefits and harms of screening and their relationship to age; underlying attitudes to the importance of cancer screening in women's lives; and use of decision aids to improve knowledge and guide decision-making.
The results of this review provide a comprehensive overview of current knowledge regarding the motivations and screening behaviour of older women about breast cancer screening which may inform policy development.
Peer Review reports
Introduction
Breast cancer is now the most commonly diagnosed cancer in the world overtaking lung cancer in 2021 [ 1 ]. Across the globe, breast cancer contributed to 25.8% of the total number of new cases of cancer diagnosed in 2020 [ 2 ] and accounts for a high disease burden for women [ 3 ]. Screening for breast cancer is an effective means of detecting early-stage cancer and has been shown to significantly improve survival rates [ 4 ]. A recent systematic review of international screening guidelines found that most countries recommend that women have biennial mammograms between the ages of 40–70 years [ 5 ] with some recommending that there should be no upper age limit [ 6 , 7 , 8 , 9 , 10 , 11 , 12 ] and others suggesting that benefits of continued screening for women over 75 are not clear [ 13 , 14 , 15 ].
Some guidelines suggest that the decision to end screening should be determined based on the individual health status of the woman, their life expectancy and current health issues [ 5 , 16 , 17 ]. This is because the benefits of mammography screening may be limited after 7 years due to existing comorbidities and limited life expectancy [ 18 , 19 , 20 , 21 ], with some jurisdictions recommending breast cancer screening for women ≥ 75 years only when life expectancy is estimated as at least 7–10 years [ 22 ]. Others have argued that decisions about continuing with screening mammography should depend on individual patient risk and health management preferences [ 23 ]. This decision is likely facilitated by a discussion between a health care provider and patient about the harms and benefits of screening outside the recommended ages [ 24 , 25 ]. While mammography may enable early detection of breast cancer, it is clear that false-positive results and overdiagnosis Footnote 1 may occur. Studies have estimated that up to 25% of breast cancer cases in the general population may be over diagnosed [ 26 , 27 , 28 ].
The risk of being diagnosed with breast cancer increases with age and approximately 80% of new cases of breast cancer in high-income countries are in women over the age of 50 [ 29 ]. The average age of first diagnosis of breast cancer in high income countries is comparable to that of Australian women which is now 61 years [ 2 , 4 , 29 ]. Studies show that women aged ≥ 75 years generally have positive attitudes to mammography screening and report high levels of perceived benefits including early detection of breast cancer and a desire to stay healthy as they age [ 21 , 30 , 31 , 32 ]. Some women aged over 74 participate, or plan to participate, in screening despite recommendations from health professionals and government guidelines advising against it [ 33 ]. Results of a recent review found that knowledge of the recommended guidelines and the potential harms of screening are limited and many older women believed that the benefits of continued screening outweighed the risks [ 30 ].
Very few studies have been undertaken to understand the motivations of women to screen or to establish screening participation rates among women aged ≥ 75 and older. This is surprising given that increasing age is recognised as a key risk factor for the development of breast cancer, and that screening is offered in many locations around the world every two years up until 74 years. The importance of this topic is high given the ambiguity around best practice for participation beyond 74 years. A preliminary search of Open Science Framework, PROSPERO, Cochrane Database of Systematic Reviews and JBI Evidence Synthesis in May 2022 did not locate any reviews on this topic.
This scoping review has allowed for the mapping of a broad range of research to explore the breadth and depth of the literature, summarize the evidence and identify knowledge gaps [ 34 , 35 ]. This information has supported the development of a comprehensive overview of current knowledge of motivations of women to screen and screening participation rates among women outside the targeted age of many international screening programs.
Materials and methods
Research question.
The research question for this scoping review was developed by applying the Population—Concept—Context (PCC) framework [ 36 ]. The current review addresses the research question “What research has been undertaken in high-income countries (context) exploring the key motivations to screen for breast cancer and screening participation (concepts) among women ≥ 75 years of age (population)?
Eligibility criteria
Participants.
Women aged ≥ 75 years were the key population. Specifically, motivations to screen and screening intention and behaviour and the variables that discriminate those who screen from those who do not (non-screeners) were utilised as the key predictors and outcomes respectively.
From a conceptual perspective it was considered that motivation led to behaviour, therefore articles that described motivation and corresponding behaviour were considered. These included articles reporting on women who use public (government funded) and private (fee for service) breast cancer screening services and those who do not use screening services (i.e., non-screeners).
The scope included high-income countries using the World Bank definition [ 37 ]. These countries have broadly similar health systems and opportunities for breast cancer screening in both public and private settings.
Types of sources
All studies reporting original research in peer-reviewed journals from January 2009 were eligible for inclusion, regardless of design. This date was selected due to an evaluation undertaken for BreastScreen Australia recommending expansion of the age group to include 70–74-year-old women [ 38 ]. This date was also indicative of international debate regarding breast cancer screening effectiveness at this time [ 39 , 40 ]. Reviews were also included, regardless of type—scoping, systematic, or narrative. Only sources published in English and available through the University’s extensive research holdings were eligible for inclusion. Ineligible materials were conference abstracts, letters to the editor, editorials, opinion pieces, commentaries, newspaper articles, dissertations and theses.
This scoping review was registered with the Open Science Framework database ( https://osf.io/fd3eh ) and followed Joanna Briggs Institute (JBI) methodology for scoping reviews [ 35 , 36 ]. Although ethics approval is not required for scoping reviews the broader study was approved by the University Ethics Committee (approval number HEC 21249).
Search strategy
A pilot search strategy was developed in consultation with an expert health librarian and tested in MEDLINE (OVID) and conducted on 3 June 2022. Articles from this pilot search were compared with seminal articles previously identified by the members of the team and used to refine the search terms. The search terms were then searched as both keywords and subject headings (e.g., MeSH) in the titles and abstracts and Boolean operators employed. A full MEDLINE search was then carried out by the librarian (see Table 1 ). This search strategy was adapted for use in each of the following databases: Cumulative Index to Nursing and Allied Health Literature (CINAHL), Medical Literature Analysis and Retrieval System Online (MEDLINE), Web of Science and PsychInfo databases. The references of included studies have been hand-searched to identify any additional evidence sources.
Study/source of evidence selection
Following the search, all identified citations were collated and uploaded into EndNote v.X20 (Clarivate Analytics, PA, USA) and duplicates removed. The resulting articles were then imported into Covidence – Cochrane’s systematic review management software [ 41 ]. Duplicates were removed once importation was complete, and title and abstract screening was undertaken against the eligibility criteria. A sample of 25 articles were assessed by all reviewers to ensure reliability in the application of the inclusion and exclusion criteria. Team discussion was used to ensure consistent application. The Covidence software supports blind reviewing with two reviewers required at each screening phase. Potentially relevant sources were retrieved in full text and were assessed against the inclusion criteria by two independent reviewers. Conflicts were flagged within the software which allows the team to discuss those that have disagreements until a consensus was reached. Reasons for exclusion of studies at full text were recorded and reported in the scoping review. The Preferred Reporting Items of Systematic Reviews extension for scoping reviews (PRISMA-ScR) checklist was used to guide the reporting of the review [ 42 ] and all stages were documented using the PRISMA-ScR flow chart [ 42 ].
Data extraction
A data extraction form was created in Covidence and used to extract study characteristics and to confirm the study’s relevance. This included specific details such as article author/s, title, year of publication, country, aim, population, setting, data collection methods and key findings relevant to the review question. The draft extraction form was modified as needed during the data extraction process.
Data analysis and presentation
Extracted data were summarised in tabular format (see Table 2 ). Consistent with the guidelines for the effective reporting of scoping reviews [ 43 ] and the JBI framework [ 35 ] the final stage of the review included thematic analysis of the key findings of the included studies. Study findings were imported into QSR NVivo with coding of each line of text. Descriptive codes reflected key aspects of the included studies related to the motivations and behaviours of women > 75 years about breast cancer screening.
In line with the reporting requirements for scoping reviews the search results for this review are presented in Fig. 1 [ 44 ].
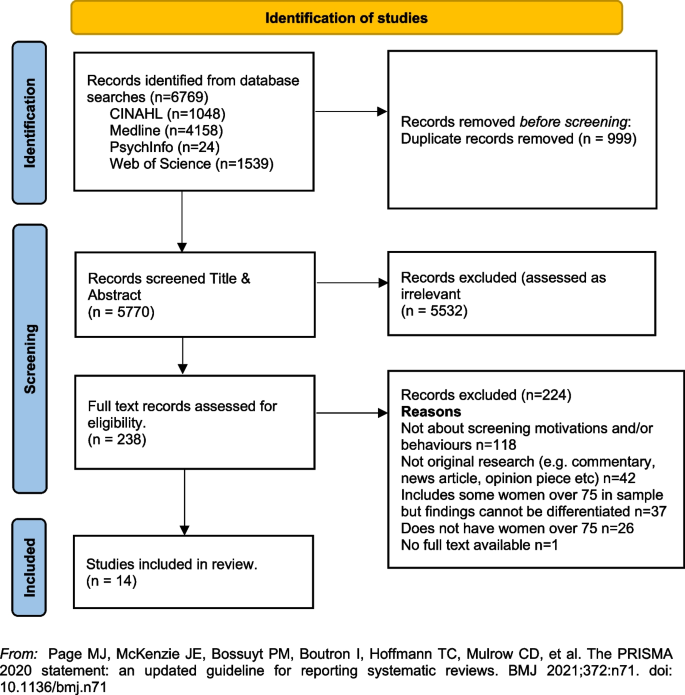
PRISMA Flowchart. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. https://doi.org/10.1136/bmj.n71
A total of fourteen [ 14 ] studies were included in the review with studies from the following countries, US n = 12 [ 33 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 ], UK n = 1 [ 23 ] and France n = 1 [ 56 ]. Sample sizes varied, with most containing fewer than 50 women ( n = 8) [ 33 , 45 , 46 , 48 , 51 , 52 , 55 ]. Two had larger samples including a French study with 136 women (a sub-set of a larger sample) [ 56 ], and one mixed method study in the UK with a sample of 26 women undertaking interviews and 479 women completing surveys [ 23 ]. One study did not report exact numbers [ 50 ]. Three studies [ 47 , 53 , 54 ] were undertaken by a group of researchers based in the US utilising the same sample of women, however each of the papers focused on different primary outcomes. The samples in the included studies were recruited from a range of locations including primary medical care clinics, specialist medical clinics, University affiliated medical clinics, community-based health centres and community outreach clinics [ 47 , 53 , 54 ].
Data collection methods varied and included: quantitative ( n = 8), qualitative ( n = 5) and mixed methods ( n = 1). A range of data collection tools and research designs were utilised; pre/post, pilot and cross-sectional surveys, interviews, and secondary analysis of existing data sets. Seven studies focused on the use of a Decision Aids (DAs), either in original or modified form, developed by Schonberg et al. [ 55 ] as a tool to increase knowledge about the harms and benefits of screening for older women [ 45 , 47 , 48 , 49 , 52 , 54 , 55 ]. Three studies focused on intention to screen [ 33 , 53 , 56 ], two on knowledge of, and attitudes to, screening [ 23 , 46 ], one on information needs relating to risks and benefits of screening discontinuation [ 51 ], and one on perceptions about discontinuation of screening and impact of social interactions on screening [ 50 ].
The three themes developed from the analysis of the included studies highlighted that decisions about screening were primarily influenced by: (1) knowledge of the benefits and harms of screening and their relationship to age; (2) underlying attitudes to the importance of cancer screening in women's lives; and (3) exposure to decision aids designed to facilitate informed decision-making. Each of these themes will be presented below drawing on the key findings of the appropriate studies. The full dataset of extracted data can be found in Table 2 .
Knowledge of the benefits and harms of screening ≥ 75 years
The decision to participate in routine mammography is influenced by individual differences in cognition and affect, interpersonal relationships, provider characteristics, and healthcare system variables. Women typically perceive mammograms as a positive, beneficial and routine component of care [ 46 ] and an important aspect of taking care of themselves [ 23 , 46 , 49 ]. One qualitative study undertaken in the US showed that few women had discussed mammography cessation or the potential harms of screening with their health care providers and some women reported they would insist on receiving mammography even without a provider recommendation to continue screening [ 46 ].
Studies suggested that ageing itself, and even poor health, were not seen as reasonable reasons for screening cessation. For many women, guidance from a health care provider was deemed the most important influence on decision-making [ 46 ]. Preferences for communication about risk and benefits were varied with one study reporting women would like to learn more about harms and risks and recommended that this information be communicated via physicians or other healthcare providers, included in brochures/pamphlets, and presented outside of clinical settings (e.g., in community-based seniors groups) [ 51 ]. Others reported that women were sometimes sceptical of expert and government recommendations [ 33 ] although some were happy to participate in discussions with health educators or care providers about breast cancer screening harms and benefits and potential cessation [ 52 ].
Underlying attitudes to the importance of cancer screening at and beyond 75 years
Included studies varied in describing the importance of screening, with some attitudes based on past attendance and some based on future intentions to screen. Three studies reported findings indicating that some women intended to continue screening after 75 years of age [ 23 , 45 , 46 ], with one study in the UK reporting that women supported an extension of the automatic recall indefinitely, regardless of age or health status. In this study, failure to invite older women to screen was interpreted as age discrimination [ 23 ]. The desire to continue screening beyond 75 was also highlighted in a study from France that found that 60% of the women ( n = 136 aged ≥ 75) intended to pursue screening in the future, and 27 women aged ≥ 75, who had never undergone mammography previously (36%), intended to do so in the future [ 56 ]. In this same study, intentions to screen varied significantly [ 56 ]. There were no sociodemographic differences observed between screened and unscreened women with regard to level of education, income, health risk behaviour (smoking, alcohol consumption), knowledge about the importance and the process of screening, or psychological features (fear of the test, fear of the results, fear of the disease, trust in screening impact) [ 56 ]. Further analysis showed that three items were statistically correlated with a higher rate of attendance at screening: (1) screening was initiated by a physician; (2) the women had a consultation with a gynaecologist during the past 12 months; and (3) the women had already undergone at least five screening mammograms. Analysis highlighted that although average income, level of education, psychological features or other types of health risk behaviours did not impact screening intention, having a mammogram previously impacted likelihood of ongoing screening. There was no information provided that explained why women who had not previously undergone screening might do so in the future.
A mixed methods study in the UK reported similar findings [ 23 ]. Utilising interviews ( n = 26) and questionnaires ( n = 479) with women ≥ 70 years (median age 75 years) the overwhelming result (90.1%) was that breast screening should be offered to all women indefinitely regardless of age, health status or fitness [ 23 ], and that many older women were keen to continue screening. Both the interview and survey data confirmed women were uncertain about eligibility for breast screening. The survey data showed that just over half the women (52.9%) were unaware that they could request mammography or knew how to access it. Key reasons for screening discontinuation were not being invited for screening (52.1%) and not knowing about self-referral (35.1%).
Women reported that not being invited to continue screening sent messages that screening was no longer important or required for this age group [ 23 ]. Almost two thirds of the women completing the survey (61.6%) said they would forget to attend screening without an invitation. Other reasons for screening discontinuation included transport difficulties (25%) and not wishing to burden family members (24.7%). By contrast, other studies have reported that women do not endorse discontinuation of screening mammography due to advancing age or poor health, but some may be receptive to reducing screening frequency on recommendation from their health care provider [ 46 , 51 ].
Use of Decision Aids (DAs) to improve knowledge and guide screening decision-making
Many women reported poor knowledge about the harms and benefits of screening with studies identifying an important role for DAs. These aids have been shown to be effective in improving knowledge of the harms and benefits of screening [ 45 , 54 , 55 ] including for women with low educational attainment; as compared to women with high educational attainment [ 47 ]. DAs can increase knowledge about screening [ 47 , 49 ] and may decrease the intention to continue screening after the recommended age [ 45 , 52 , 54 ]. They can be used by primary care providers to support a conversation about breast screening intention and reasons for discontinuing screening. In one pilot study undertaken in the US using a DA, 5 of the 8 women (62.5%) indicated they intended to continue to receive mammography; however, 3 participants planned to get them less often [ 45 ]. When asked whether they thought their physician would want them to get a mammogram, 80% said “yes” on pre-test; this figure decreased to 62.5% after exposure to the DA. This pilot study suggests that the use of a decision-aid may result in fewer women ≥ 75 years old continuing to screen for breast cancer [ 45 ].
Similar findings were evident in two studies drawing on the same data undertaken in the US [ 48 , 53 ]. Using a larger sample ( n = 283), women’s intentions to screen prior to a visit with their primary care provider and then again after exposure to the DA were compared. Results showed that 21.7% of women reduced their intention to be screened, 7.9% increased their intentions to be screened, and 70.4% did not change. Compared to those who had no change or increased their screening intentions, women who had a decrease in screening intention were significantly less likely to receive screening after 18 months. Generally, studies have shown that women aged 75 and older find DAs acceptable and helpful [ 47 , 48 , 49 , 55 ] and using them had the potential to impact on a women’s intention to screen [ 55 ].
Cadet and colleagues [ 49 ] explored the impact of educational attainment on the use of DAs. Results highlight that education moderates the utility of these aids; women with lower educational attainment were less likely to understand all the DA’s content (46.3% vs 67.5%; P < 0.001); had less knowledge of the benefits and harms of mammography (adjusted mean ± standard error knowledge score, 7.1 ± 0.3 vs 8.1 ± 0.3; p < 0.001); and were less likely to have their screening intentions impacted (adjusted percentage, 11.4% vs 19.4%; p = 0.01).
This scoping review summarises current knowledge regarding motivations and screening behaviours of women over 75 years. The findings suggest that awareness of the importance of breast cancer screening among women aged ≥ 75 years is high [ 23 , 46 , 49 ] and that many women wish to continue screening regardless of perceived health status or age. This highlights the importance of focusing on motivation and screening behaviours and the multiple factors that influence ongoing participation in breast screening programs.
The generally high regard attributed to screening among women aged ≥ 75 years presents a complex challenge for health professionals who are focused on potential harm (from available national and international guidelines) in ongoing screening for women beyond age 75 [ 18 , 20 , 57 ]. Included studies highlight that many women relied on the advice of health care providers regarding the benefits and harms when making the decision to continue breast screening [ 46 , 51 , 52 ], however there were some that did not [ 33 ]. Having a previous pattern of screening was noted as being more significant to ongoing intention than any other identified socio-demographic feature [ 56 ]. This is perhaps because women will not readily forgo health care practices that they have always considered important and that retain ongoing importance for the broader population.
For those women who had discontinued screening after the age of 74 it was apparent that the rationale for doing so was not often based on choice or receipt of information, but rather on factors that impact decision-making in relation to screening. These included no longer receiving an invitation to attend, transport difficulties and not wanting to be a burden on relatives or friends [ 23 , 46 , 51 ]. Ongoing receipt of invitations to screen was an important aspect of maintaining a capacity to choose [ 23 ]. This was particularly important for those women who had been regular screeners.
Women over 75 require more information to make decisions regarding screening [ 23 , 52 , 54 , 55 ], however health care providers must also be aware that the element of choice is important for older women. Having a capacity to choose avoids any notion of discrimination based on age, health status, gender or sociodemographic difference and acknowledges the importance of women retaining control over their health [ 23 ]. It was apparent that some women would choose to continue screening at a reduced frequency if this option was available and that women should have access to information facilitating self-referral [ 23 , 45 , 46 , 51 , 56 ].
Decision-making regarding ongoing breast cancer screening has been facilitated via the use of Decision Aids (DAs) within clinical settings [ 54 , 55 ]. While some studies suggest that women will make a decision regardless of health status, the use of DAs has impacted women’s decision to screen. While this may have limited benefit for those of lower educational attainment [ 48 ] they have been effective in improving knowledge relating to harms and benefits of screening particularly where they have been used to support a conversation with women about the value of screening [ 54 , 55 , 56 ].
Women have identified challenges in engaging in conversations with health care providers regarding ongoing screening, because providers frequently draw on projections of life expectancy and over-diagnosis [ 17 , 51 ]. As a result, these conversations about screening after age 75 years often do not occur [ 46 ]. It is likely that health providers may need more support and guidance in leading these conversations. This may be through the use of DAs or standardised checklists. It may be possible to incorporate these within existing health preventive measures for this age group. The potential for advice regarding ongoing breast cancer screening to be available outside of clinical settings may provide important pathways for conversations with women regarding health choices. Provision of information and advice in settings such as community based seniors groups [ 51 ] offers a potential platform to broaden conversations and align sources of information, not only with health professionals but amongst women themselves. This may help to address any misconception regarding eligibility and access to services [ 23 ]. It may also be aligned with other health promotion and lifestyle messages provided to this age group.
Limitations of the review
The searches that formed the basis of this review were carried in June 2022. Although the search was comprehensive, we have only captured those studies that were published in the included databases from 2009. There may have been other studies published outside of these periods. We also limited the search to studies published in English with full-text availability.
The emphasis of a scoping review is on comprehensive coverage and synthesis of the key findings, rather than on a particular standard of evidence and, consequently a quality assessment of the included studies was not undertaken. This has resulted in the inclusion of a wide range of study designs and data collection methods. It is important to note that three studies included in the review drew on the same sample of women (283 over > 75)[ 49 , 53 , 54 ]. The results of this review provide valuable insights into motivations and behaviours for breast cancer screening for older women, however they should be interpreted with caution given the specific methodological and geographical limitations.
Conclusion and recommendations
This scoping review highlighted a range of key motivations and behaviours in relation to breast cancer screening for women ≥ 75 years of age. The results provide some insight into how decisions about screening continuation after 74 are made and how informed decision-making can be supported. Specifically, this review supports the following suggestions for further research and policy direction:
Further research regarding breast cancer screening motivations and behaviours for women over 75 would provide valuable insight for health providers delivering services to women in this age group.
Health providers may benefit from the broader use of decision aids or structured checklists to guide conversations with women over 75 regarding ongoing health promotion/preventive measures.
Providing health-based information in non-clinical settings frequented by women in this age group may provide a broader reach of information and facilitate choices. This may help to reduce any perception of discrimination based on age, health status or socio-demographic factors.
Availability of data and materials
All data generated or analysed during this study is included in this published article (see Table 2 above).
Cancer Australia, in their 2014 position statement, define “overdiagnosis” in the following way. ‘’Overdiagnosis’ from breast screening does not refer to error or misdiagnosis, but rather refers to breast cancer diagnosed by screening that would not otherwise have been diagnosed during a woman’s lifetime. “Overdiagnosis” includes all instances where cancers detected through screening (ductal carcinoma in situ or invasive breast cancer) might never have progressed to become symptomatic during a woman’s life, i.e., cancer that would not have been detected in the absence of screening. It is not possible to precisely predict at diagnosis, to which cancers overdiagnosis would apply.” (accessed 22. nd August 2022; https://www.canceraustralia.gov.au/resources/position-statements/overdiagnosis-mammographic-screening ).
World Health Organization. Breast Cancer Geneva: WHO; 2021 [Available from: https://www.who.int/news-room/fact-sheets/detail/breast-cancer#:~:text=Reducing%20global%20breast%20cancer%20mortality,and%20comprehensive%20breast%20cancer%20management .
International Agency for Research on Cancer (IARC). IARC Handbooks on Cancer Screening: Volume 15 Breast Cancer Geneva: IARC; 2016 [Available from: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Handbooks-Of-Cancer-Prevention/Breast-Cancer-Screening-2016 .
Australian Institute of Health and Welfare. Cancer in Australia 2021 [Available from: https://www.canceraustralia.gov.au/cancer-types/breast-cancer/statistics .
Breast Cancer Network Australia. Current breast cancer statistics in Australia 2020 [Available from: https://www.bcna.org.au/media/7111/bcna-2019-current-breast-cancer-statistics-in-australia-11jan2019.pdf .
Ren W, Chen M, Qiao Y, Zhao F. Global guidelines for breast cancer screening: A systematic review. The Breast. 2022;64:85–99.
Article PubMed PubMed Central Google Scholar
Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194–220.
Article CAS PubMed Google Scholar
Hamashima C, Hattori M, Honjo S, Kasahara Y, Katayama T, Nakai M, et al. The Japanese guidelines for breast cancer screening. Jpn J Clin Oncol. 2016;46(5):482–92.
Article PubMed Google Scholar
Bevers TB, Helvie M, Bonaccio E, Calhoun KE, Daly MB, Farrar WB, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Net. 2018;16(11):1362–89.
Article Google Scholar
He J, Chen W, Li N, Shen H, Li J, Wang Y, et al. China guideline for the screening and early detection of female breast cancer (2021, Beijing). Zhonghua Zhong liu za zhi [Chinese Journal of Oncology]. 2021;43(4):357–82.
CAS PubMed Google Scholar
Cancer Australia. Early detection of breast cancer 2021 [cited 2022 25 July]. Available from: https://www.canceraustralia.gov.au/resources/position-statements/early-detection-breast-cancer .
Schünemann HJ, Lerda D, Quinn C, Follmann M, Alonso-Coello P, Rossi PG, et al. Breast Cancer Screening and Diagnosis: A Synopsis of the European Breast Guidelines. Ann Intern Med. 2019;172(1):46–56.
World Health Organization. WHO Position Paper on Mammography Screening Geneva WHO. 2016.
Google Scholar
Lansdorp-Vogelaar I, Gulati R, Mariotto AB. Personalizing age of cancer screening cessation based on comorbid conditions: model estimates of harms and benefits. Ann Intern Med. 2014;161:104.
Lee CS, Moy L, Joe BN, Sickles EA, Niell BL. Screening for Breast Cancer in Women Age 75 Years and Older. Am J Roentgenol. 2017;210(2):256–63.
Broeders M, Moss S, Nystrom L. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(suppl 1):14.
Oeffinger KC, Fontham ETH, Etzioni R, Herzig A, Michaelson JS, Shih YCT, et al. Breast cancer screening for women at average risk: 2015 Guideline update from the American cancer society. JAMA - Journal of the American Medical Association. 2015;314(15):1599–614.
Walter LC, Schonberg MA. Screening mammography in older women: a review. JAMA. 2014;311:1336.
Article CAS PubMed PubMed Central Google Scholar
Braithwaite D, Walter LC, Izano M, Kerlikowske K. Benefits and harms of screening mammography by comorbidity and age: a qualitative synthesis of observational studies and decision analyses. J Gen Intern Med. 2016;31:561.
Braithwaite D, Mandelblatt JS, Kerlikowske K. To screen or not to screen older women for breast cancer: a conundrum. Future Oncol. 2013;9(6):763–6.
Demb J, Abraham L, Miglioretti DL, Sprague BL, O’Meara ES, Advani S, et al. Screening Mammography Outcomes: Risk of Breast Cancer and Mortality by Comorbidity Score and Age. Jnci-Journal of the National Cancer Institute. 2020;112(6):599–606.
Demb J, Akinyemiju T, Allen I, Onega T, Hiatt RA, Braithwaite D. Screening mammography use in older women according to health status: a systematic review and meta-analysis. Clin Interv Aging. 2018;13:1987–97.
Qaseem A, Lin JS, Mustafa RA, Horwitch CA, Wilt TJ. Screening for Breast Cancer in Average-Risk Women: A Guidance Statement From the American College of Physicians. Ann Intern Med. 2019;170(8):547–60.
Collins K, Winslow M, Reed MW, Walters SJ, Robinson T, Madan J, et al. The views of older women towards mammographic screening: a qualitative and quantitative study. Br J Cancer. 2010;102(10):1461–7.
Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605–13.
Hersch J, Jansen J, Barratt A, Irwig L, Houssami N, Howard K, et al. Women’s views on overdiagnosis in breast cancer screening: a qualitative study. BMJ : British Medical Journal. 2013;346:f158.
De Gelder R, Heijnsdijk EAM, Van Ravesteyn NT, Fracheboud J, Draisma G, De Koning HJ. Interpreting overdiagnosis estimates in population-based mammography screening. Epidemiol Rev. 2011;33(1):111–21.
Monticciolo DL, Helvie MA, Edward HR. Current issues in the overdiagnosis and overtreatment of breast cancer. Am J Roentgenol. 2018;210(2):285–91.
Shepardson LB, Dean L. Current controversies in breast cancer screening. Semin Oncol. 2020;47(4):177–81.
National Cancer Control Centre. Cancer incidence in Australia 2022 [Available from: https://ncci.canceraustralia.gov.au/diagnosis/cancer-incidence/cancer-incidence .
Austin JD, Shelton RC, Lee Argov EJ, Tehranifar P. Older Women’s Perspectives Driving Mammography Screening Use and Overuse: a Narrative Review of Mixed-Methods Studies. Current Epidemiology Reports. 2020;7(4):274–89.
Austin JD, Tehranifar P, Rodriguez CB, Brotzman L, Agovino M, Ziazadeh D, et al. A mixed-methods study of multi-level factors influencing mammography overuse among an older ethnically diverse screening population: implications for de-implementation. Implementation Science Communications. 2021;2(1):110.
Demb J, Allen I, Braithwaite D. Utilization of screening mammography in older women according to comorbidity and age: protocol for a systematic review. Syst Rev. 2016;5(1):168.
Housten AJ, Pappadis MR, Krishnan S, Weller SC, Giordano SH, Bevers TB, et al. Resistance to discontinuing breast cancer screening in older women: A qualitative study. Psychooncology. 2018;27(6):1635–41.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
Peters M, Godfrey C, McInerney P, Munn Z, Tricco A, Khalil HAE, et al. Chapter 11: Scoping reviews. JBI Manual for Evidence Synthesis 2020 [Available from: https://jbi-global-wiki.refined.site/space/MANUAL .
Peters MD, Godfrey C, McInerney P, Khalil H, Larsen P, Marnie C, et al. Best practice guidance and reporting items for the development of scoping review protocols. JBI evidence synthesis. 2022;20(4):953–68.
Fantom NJ, Serajuddin U. The World Bank’s classification of countries by income. World Bank Policy Research Working Paper; 2016.
Book Google Scholar
BreastScreen Australia Evaluation Taskforce. BreastScreen Australia Evaluation. Evaluation final report: Screening Monograph No 1/2009. Canberra; Australia Australian Government Department of Health and Ageing; 2009.
Nelson HD, Cantor A, Humphrey L. Screening for breast cancer: a systematic review to update the 2009 U.S. Preventive Services Task Force recommendation2016.
Woolf SH. The 2009 breast cancer screening recommendations of the US Preventive Services Task Force. JAMA. 2010;303(2):162–3.
Covidence systematic review software. [Internet]. Veritas-Health-Innovation 2020. Available from: https://www.covidence.org/ .
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467–73.
Tricco AC, Lillie E, Zarin W, O’Brien K, Colquhoun H, Kastner M, et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med Res Methodol. 2016;16(1):15.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Beckmeyer A, Smith RM, Miles L, Schonberg MA, Toland AE, Hirsch H. Pilot Evaluation of Patient-centered Survey Tools for Breast Cancer Screening Decision-making in Women 75 and Older. Health Behavior and Policy Review. 2020;7(1):13–8.
Brotzman LE, Shelton RC, Austin JD, Rodriguez CB, Agovino M, Moise N, et al. “It’s something I’ll do until I die”: A qualitative examination into why older women in the U.S. continue screening mammography. Canc Med. 2022;11(20):3854–62.
Article CAS Google Scholar
Cadet T, Pinheiro A, Karamourtopoulos M, Jacobson AR, Aliberti GM, Kistler CE, et al. Effects by educational attainment of a mammography screening patient decision aid for women aged 75 years and older. Cancer. 2021;127(23):4455–63.
Cadet T, Aliberti G, Karamourtopoulos M, Jacobson A, Gilliam EA, Primeau S, et al. Evaluation of a mammography decision aid for women 75 and older at risk for lower health literacy in a pretest-posttest trial. Patient Educ Couns. 2021;104(9):2344–50.
Cadet T, Aliberti G, Karamourtopoulos M, Jacobson A, Siska M, Schonberg MA. Modifying a mammography decision aid for older adult women with risk factors for low health literacy. Health Lit Res Prac. 2021;5(2):e78–90.
Gray N, Picone G. Evidence of Large-Scale Social Interactions in Mammography in the United States. Atl Econ J. 2018;46(4):441–57.
Hoover DS, Pappadis MR, Housten AJ, Krishnan S, Weller SC, Giordano SH, et al. Preferences for Communicating about Breast Cancer Screening Among Racially/Ethnically Diverse Older Women. Health Commun. 2019;34(7):702–6.
Salzman B, Bistline A, Cunningham A, Silverio A, Sifri R. Breast Cancer Screening Shared Decision-Making in Older African-American Women. J Natl Med Assoc. 2020;112(5):556–60.
PubMed Google Scholar
Schoenborn NL, Pinheiro A, Kistler CE, Schonberg MA. Association between Breast Cancer Screening Intention and Behavior in the Context of Screening Cessation in Older Women. Med Decis Making. 2021;41(2):240–4.
Schonberg MA, Kistler CE, Pinheiro A, Jacobson AR, Aliberti GM, Karamourtopoulos M, et al. Effect of a Mammography Screening Decision Aid for Women 75 Years and Older: A Cluster Randomized Clinical Trial. JAMA Intern Med. 2020;180(6):831–42.
Schonberg MA, Hamel MB, Davis RB. Development and evaluation of a decision aid on mammography screening for women 75 years and older. JAMA Intern Med. 2014;174:417.
Eisinger F, Viguier J, Blay J-Y, Morère J-F, Coscas Y, Roussel C, et al. Uptake of breast cancer screening in women aged over 75years: a controversy to come? Eur J Cancer Prev. 2011;20(Suppl 1):S13-5.
Schonberg MA, Breslau ES, McCarthy EP. Targeting of Mammography Screening According to Life Expectancy in Women Aged 75 and Older. J Am Geriatr Soc. 2013;61(3):388–95.
Download references
Acknowledgements
We would like to acknowledge Ange Hayden-Johns (expert librarian) who assisted with the development of the search criteria and undertook the relevant searches and Tejashree Kangutkar who assisted with some of the Covidence work.
This work was supported by funding from the Australian Government Department of Health and Aged Care (ID: Health/20–21/E21-10463).
Author information
Authors and affiliations.
Violet Vines Centre for Rural Health Research, La Trobe Rural Health School, La Trobe University, P.O. Box 199, Bendigo, VIC, 3552, Australia
Virginia Dickson-Swift, Joanne Adams & Evelien Spelten
Care Economy Research Institute, La Trobe University, Wodonga, Australia
Irene Blackberry
Olivia Newton-John Cancer Wellness and Research Centre, Austin Health, Melbourne, Australia
Carlene Wilson & Eva Yuen
Melbourne School of Population and Global Health, Melbourne University, Melbourne, Australia
Carlene Wilson
School of Psychology and Public Health, La Trobe University, Bundoora, Australia
Institute for Health Transformation, Deakin University, Burwood, Australia
Centre for Quality and Patient Safety, Monash Health Partnership, Monash Health, Clayton, Australia
You can also search for this author in PubMed Google Scholar
Contributions
VDS conceived and designed the scoping review. VDS & JA developed the search strategy with librarian support, and all authors (VDS, JA, ES, IB, CW, EY) participated in the screening and data extraction stages and assisted with writing the review. All authors provided editorial support and read and approved the final manuscript prior to submission.
Corresponding author
Correspondence to Joanne Adams .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethics approval and consent to participate
Ethics approval and consent to participate was not required for this study.
Consent for publication
Consent for publication was not required for this study.
Competing interest
The authors declare they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Dickson-Swift, V., Adams, J., Spelten, E. et al. Breast cancer screening motivation and behaviours of women aged over 75 years: a scoping review. BMC Women's Health 24 , 256 (2024). https://doi.org/10.1186/s12905-024-03094-z
Download citation
Received : 06 September 2023
Accepted : 15 April 2024
Published : 24 April 2024
DOI : https://doi.org/10.1186/s12905-024-03094-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Mammography
- Older women
- Scoping review
BMC Women's Health
ISSN: 1472-6874
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Screening for Breast Cancer: A Comparative Review of Guidelines
- Katsika, Laskarina
- Boureka, Eirini
- Kalogiannidis, Ioannis
- Tsakiridis, Ioannis
- Tirodimos, Ilias
- Lallas, Konstantinos
- Tsimtsiou, Zoi
- Dagklis, Themistoklis
Breast cancer is the most common malignancy diagnosed in the female population worldwide and the leading cause of death among perimenopausal women. Screening is essential, since earlier detection in combination with improvements in breast cancer treatment can reduce the associated mortality. The aim of this study was to review and compare the recommendations from published guidelines on breast cancer screening. A total of 14 guidelines on breast cancer screening issued between 2014 and 2022 were identified. A descriptive review of relevant guidelines by the World Health Organization (WHO), the U.S. Preventive Services Task Force (USPSTF), the American Cancer Society (ACS), the National Comprehensive Cancer Network (NCCN), the American College of Obstetricians and Gynecologists (ACOG), the American Society of Breast Surgeons (ASBrS), the American College of Radiology (ACR), the Task Force on Preventive Health Care (CTFPHC), the European Commission Initiative on Breast Cancer (ECIBC), the European Society for Medical Oncology (ESMO), the Royal Australian College of General Practitioners (RACGP) and the Japanese Journal of Clinical Oncology (JJCO) for women both at average and high-risk was carried out. There is a consensus among all the reviewed guidelines that mammography is the gold standard screening modality for average-risk women. For this risk group, most of the guidelines suggest annual or biennial mammographic screening at 40–74 years, while screening should particularly focus at 50–69 years. Most of the guidelines suggest that the age limit to stop screening should be determined based on the women's health status and life expectancy. For women at high-risk, most guidelines recommend the use of annual mammography or magnetic resonance imaging, while the starting age should be earlier than the average-risk group, depending on the risk factor. There is discrepancy among the recommendations regarding the age at onset of screening in the various high-risk categories. The development of consistent international practice protocols for the most appropriate breast cancer screening programs seems of major importance to reduce mortality rates and safely guide everyday clinical practice.
- breast cancer;
- mammography;
- guidelines;
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- News & Views
- Breast cancer...
Breast cancer screening from age 40 in the US
- Related content
- Peer review
- Katy JL Bell , professor 1 2 ,
- Brooke Nickel , senior research fellow 1 2 ,
- Thanya Pathirana , senior lecturer 2 3 ,
- Mitzi Blennerhassett , patient activist 4 ,
- Stacy Carter , professor 2 5
- 1 Sydney School of Public Health, University of Sydney, NSW, Australia
- 2 Wiser Healthcare Research Collaboration, Australia
- 3 School of Medicine and Dentistry, Griffith University, Sunshine Coast, QLD, Australia
- 4 Patient representative, York, UK
- 5 Australian Centre for Health Engagement, Evidence and Values, School of Health and Society, University of Wollongong, NSW, Australia
- Correspondence to: K J L Bell katy.bell{at}sydney.edu.au
The new recommendation could cause more harm than benefit
The US Preventive Services Task Force has updated its recommendation for the age when all women should start mammography screening, lowering it from 50 to 40. 1 This change immediately affects more than 20 million American women and other people assigned female at birth who are aged 40-49, 2 with repercussions far beyond the US.
Such a momentous change should reflect new randomised trial evidence or concerning cancer mortality trends. But no such trial evidence was found in the commissioned evidence report, 3 and breast cancer mortality has been decreasing, especially among women under 50. 4 The new recommendation seems to be based on two, inter-related, considerations. The first is recognition of the inequality in breast cancer mortality between Black and white US women, and a commitment to reduce this. The second is statistical modelling of a hypothetical population that found starting screening at 40 would reduce breast cancer mortality, especially among Black women. 5
The need to make health policy and systems antiracist and more equitable is urgent and compelling. But there is little empirical evidence that lowering the screening age will achieve this. We agree with others’ concerns 4 6 about the task force’s increasing reliance on modelling over empirical evidence. The modelling reported a more favourable benefit to harm ratio for all population groups than the trial evidence and made several assumptions that may not represent reality, 7 8 including few non-progressive or rapidly growing cancers (where screening has no benefit), high adherence, and large mortality benefits, especially for Black women.
The racial inequality in US breast cancer mortality has been observed since wide adoption of screening mammography (and adjuvant endocrine therapy) in the 1980s. 9 Screening primarily benefits women with cancers that are hormone receptor (HR) positive; HR negative tumours are more aggressive and tend to be diagnosed at later stages, among younger women, and missed by mammography screening. 6 HR negative tumours are more common in Black women for hereditary reasons and because of social determinants of health. 9 Instead of expanding mammography screening to younger women, initiatives are needed that tackle the systemic injustices driving racial inequality in breast cancer care, especially in access to high quality, timely, and effective care and treatment. 4 9
Uncertain evidence
Globally, the US may be an outlier in making a strong recommendation to start population mammography screening at age 40 rather than age 50. 10 11 12 The task force’s evidence report found uncertain evidence of a potential mortality benefit in women aged 40-49: the 95% confidence interval spanned from six more deaths to 89 fewer deaths per 100 000 screened. 3 None of the included trials indicated significantly reduced breast cancer mortality with screening, including the UK Age trial, the largest (n=160 921) and most recent trial specifically designed to determine the effectiveness of screening women in their 40s. 13 This small and uncertain benefit needs to be considered against harms.
False positive mammography rates were highest among those aged 40 to 49 years: 12 120 (95% CI 10 560 to 13 870) per 100 000 screened. Recommendations for additional diagnostic imaging were also highest in 40-49 year olds: 12 490 (10 930 to 14 230) per 100 000. Many women will also have clinical consultations and procedures such as surgical biopsies, creating appreciable costs to the health system as well as potential out-of-pocket costs. 14 Adverse psychosocial consequences, such as anxiety and finding time in busy lives for follow-up, pose additional burdens.
Trial estimates of overdiagnosis (cancers that would have never have caused symptoms or death if left undetected and untreated) ranged from 11% to 22% of detected cancers. Most of those overdiagnosed will also be overtreated with surgery (with or without adjuvant radiotherapy) and hormone therapy. 15 16 They will not benefit from this, but they may be harmed—through adverse effects of surgery and hormone therapy, and through increased risk of coronary heart disease and other cancers after radiotherapy. 15 Finally, although there were no trial data on effects of mammography radiation, a modelling report found that there could be seven additional radiation induced breast cancer deaths per 100 000 women with biennial screening starting at age 40 (12 deaths) rather than age 50 (5 deaths). 17
Better routes to equity
Decreasing the age for mammography screening offers only possible marginal health benefit for individuals, with substantially increased risk of harm. Screening is also resource intensive for health systems, using up funding, 14 clinician hours, 18 and facilities, all of which contribute to healthcare’s carbon footprint. 19 This diverts resources that would be better used elsewhere—for example, in improving access to effective cancer treatments and care in underserved communities. 20 The opportunity costs are even more pressing in low and middle income countries. 20 21
Health systems globally need transformation to remove systematic racism and discrimination, and tackle health inequalities. Instead of adopting the new US recommendations, policy makers should work with communities to co-design initiatives that tackle the root causes of the racial inequality in breast cancer care for Black women and other underserved groups. Patients 16 and the public 22 need to be empowered and actively supported to understand, be involved in, and have an influence on, practice and policy decisions—including the design of screening programmes.
Competing interests: We have read and understood BMJ policy on declaration of interests and declare the following: MB has campaigned against the Age X trial and breast screening, although no finance has been involved.
Provenance and peer review: Commissioned; externally peer reviewed.
- US Preventive Services Task Force, Nicholson SM, Silverstein M, et al
- ↵ United States Census Bureau. Age and sex composition in the United States: 2021. https://www.census.gov/data/tables/2021/demo/age-and-sex/2021-age-sex-composition.html .
- Henderson JT ,
- Webber EM ,
- Weyrich MS ,
- Woloshin S ,
- Jørgensen KJ ,
- Trentham-Dietz A ,
- Chapman CH ,
- Jayasekera J ,
- Elmore JG ,
- Kalager M ,
- Barratt A ,
- ↵ Canadian Task Force on Preventive health Care. Breast cancer (update) - draft recommendations. 2024. https://canadiantaskforce.ca/breast-cancer-update-draft-recommendations/ .
- Houssami N ,
- Clemson M ,
- ↵ Jeffers S. Saying “no” to overtreatment—not as easy as it sounds. Health Sense, 2024 https://www.healthsense-uk.org/publications/newsletter/newsletter-125/384-125-jeffers.html .
- Miglioretti DL ,
- van den Broek JJ ,
- Johansson M ,
- Albarqouni L ,
- Abukmail E ,
- MohammedAli M ,
- ↵ World Health Organization. WHO position paper on mammography screening. 2014. https://www.who.int/publications/i/item/9789241507936 .
- Degeling C ,
- Carter SM ,
- Rychetnik L
Breast cancer screening a literature review
- Mai Alsammak Department of Family Medicine, Madinat Khalifa Health Centre, Primary Health Care Corporation, Doha, Qatar
- Marwa Khattabi Department of Family Medicine, Madinat Khalifa Health Centre, Primary Health Care Corporation, Doha, Qatar
This article is looking at literature on breast cancer screening. Being the most common cancer worldwide and a leading cause of death, screening asymptotic women leads to early detection hence early treatment and with advances in treatments, breast cancer has better survival outcomes.
Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. The Breast. 2022;66:15-23.
Peto R, Boreham J, Clarke M, Davies C, Beral V. UK and USA breast cancer deaths down 25% in year 2000 at ages 20-69 years. Lancet. 2000;355(9217):1822.
Hashim MJ, Al-Shamsi FA, Al-Marzooqi NA, Al-Qasemi SS, Mokdad AH, Khan G. Burden of breast cancer in the Arab world: findings from Global Burden of Disease. J Epidemiol Global Heal. 2018;8(1-2):54.
Ouanhnon L, Bugat MER, Lamy S, Druel V, Delpierre C, Grosclaude P. Social and territorial inequalities in breast and cervical cancers screening uptake: a cross-sectional study in France. BMJ Open. 2022;12(2):e055363.
Siu AL, US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Internal Med. 2016;164(4):279-96.
Miller AB, Wall C, Baines CJ, Sun P, To T, Narod SA. Twenty-five-year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study Randomised Screening trial. BMJ. 2014;348.
Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer screening: an independent review. Brit J Cancer. 2013;108(11):2205-40.
Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Internal Med. 2009;151(10):727-37.
Nyström L, Andersson I, Bjurstam N, Frisell J, Nordenskjöld B, Rutqvist LE. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet. 2002;359(9310):909-19.
Autier P, Koechlin A, Smans M, Vatten L, Boniol M. Mammography screening and breast cancer mortality in Sweden. J National Cancer Inst. 2012;104(14):1080-93.
Gøtzsche PC, Jørgensen KJ, Cochrane Breast Cancer Group. Screening for breast cancer with mammography. Cochrane Database Systematic Rev. 1996;2013(6).
Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Eng J Med. 2005;353(17):1784-92.
Weedon-Fekjær H, Romundstad PR, Vatten LJ. Modern mammography screening and breast cancer mortality: population study. BMJ 2014;348.
Otto SJ, Fracheboud J, Verbeek AL, Boer R, Reijerink-Verheij JC, Otten JD et al. Mammography screening and risk of breast cancer death: a population-based case–control study. Cancer Epidemiol Biomarkers Prevention. 2012;21(1):66-73.
Elmore JG, Reisch LM, Barton MB, Barlow WE, Rolnick S, Harris EL, et al. Efficacy of breast cancer screening in the community according to risk level. J National Cancer Inst. 2005;97(14):1035-43.
Lauby-Secretan B, Scoccianti C, Loomis D, Benbrahim-Tallaa L, Bouvard V, Bianchini F, et al. Breast-cancer screening-viewpoint of the IARC Working Group. N Eng J Med. 2015;372(24):2353-8.
Brown ML, Houn F, Sickles EA, Kessler LG. Screening mammography in community practice: positive predictive value of abnormal findings and yield of follow-up diagnostic procedures. Am J Roentgenol. 1995;165(6):1373-7.
Hubbard RA, Kerlikowske K, Flowers CI, Yankaskas BC, Zhu W, Miglioretti DL. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Internal Med. 2011;155(8):481-92.
Christiansen CL, Wang F, Barton MB, Kreuter W, Elmore JG, Gelfand AE, et al. Predicting the cumulative risk of false-positive mammograms. J National Cancer Inst. 2000;92(20):1657-66.
Barton MB, Morley DS, Moore S, Allen JD, Kleinman KP, Emmons KM, et al. Decreasing women's anxieties after abnormal mammograms: a controlled trial. J National Cancer Inst. 2004;96(7):529-38.
Coldman A, Phillips N. Incidence of breast cancer and estimates of overdiagnosis after the initiation of a population-based mammography screening program. CMAJ. 2013;185(10):E492-8.
Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer screening: an independent review. Bri J Cancer. 2013;108(11):2205-40.
Welch HG, Prorok PC, O’Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Eng J Med. 2016;375(15):1438-47.
Wadhwa A, Sullivan JR, Gonyo MB. Missed breast cancer: what can we learn? Curr Prob Diagnostic Radiol. 2016;45(6):402-19.
Lamb LR, Mohallem Fonseca M, Verma R, Seely JM. Missed breast cancer: effects of subconscious bias and lesion characteristics. Radiographics, 2020;40(4):941-60.
Lehman CD, Arao RF, Sprague BL, Lee JM, Buist DS, Kerlikowske K, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49-58.
Mamdouh HM, El-Mansy H, Kharboush IF, Ismail HM, Tawfik MM, El-Baky MA, et al. Barriers to breast cancer screening among a sample of Egyptian females. J Family Community Med. 2014;21(2):119.
Tsapatsaris A, Babagbemi K, Reichman MB. Barriers to breast cancer screening are worsened amidst COVID-19 pandemic: A review. Clin Imaging, 2022;82:224-7.
Alter RC, Yaffe MJ. Breast cancer screening and anxiety. J Breast Imaging. 2021;3(3):273-5.
Bonfill Cosp X, Marzo Castillejo M, Pladevall Vila M, Marti J, Emparanza JI, Cochrane Breast Cancer Group. Strategies for increasing the participation of women in community breast cancer screening. Cochrane Database Systematic Rev. 1996;2016(9).
Brown SL, Gibney TM, Tarling R. Busy lifestyles and mammography screening: time pressure and women’s reattendance likelihood. Psychol Health. 2013;28(8):928-38.
Battaglia TA, Roloff K, Posner MA, Freund KM. Improving follow‐up to abnormal breast cancer screening in an urban population: a patient navigation intervention. Cancer Interdisciplinary Int J Am Cancer Society. 2007;109(S2):359-67.
Naz MSG, Simbar M, Fakari FR, Ghasemi V. Effects of model-based interventions on breast cancer screening behavior of women: a systematic review. Asian Pacific J Cancer Prevent 2018;19(8):2031.
Deppen SA, Aldrich MC, Hartge P, Berg CD, Colditz GA, Petitti DB, et al. Cancer screening: the journey from epidemiology to policy. Ann Epidemiol. 2012;22(6):439-45.
How to Cite
- Endnote/Zotero/Mendeley (RIS)
Make a Submission
Information.
- For Readers
- For Authors
- For Librarians
Current Issue
International Journal Of Community Medicine And Public Health. Copyright © 2024.
Print ISSN: 2394-6032 | Online ISSN: 2394-6040
[email protected] , [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 03 July 2024
Breast cancer screening patterns and associated factors in Iranian women over 40 years
- Elham Seyedkanani 1 ,
- Mina Hosseinzadeh 1 ,
- Mojgan Mirghafourvand 2 &
- Leila Sheikhnezhad 1
Scientific Reports volume 14 , Article number: 15274 ( 2024 ) Cite this article
178 Accesses
Metrics details
- Health care
Screening is a key component of breast cancer early detection programs that can considerably reduce relevant mortality rates. The purpose of this study was to determine the breast cancer screening behavioral patterns and associated factors in women over 40 years of age. In this descriptive‑analytical cross‑sectional study, 372 over 40 years of age women visiting health centers in Tabriz, Iran, in 2023 were enrolled using cluster sampling. The data were collected using the sociodemographic characteristics questionnaire, breast cancer perception scale, health literacy for Iranian adults scale, and the Breast Cancer Screening Behavior Checklist. The obtained data were analyzed in SPSS version 16 using descriptive statistics (frequency, percentage, mean, and standard deviation) and inferential statistics (univariate and multivariate logistic regression analyses). In total, 68.3% of all participants performed breast self‑examination (BSE) (9.9% regularly, once per month), 60.2% underwent clinical breast examination (CBE) (8.9% regularly, twice per year), 51.3% underwent mammography (12.3% regularly, once per year), and 36.2% underwent sonography (3.8% regularly, twice per year). The findings also showed that women with benign breast diseases were more likely to undergo CBE (OR = 8.49; 95% CI 2.55 to 28.21; P < 0.001), mammography (OR = 8.84; 95% CI 2.98 to 10; P < 0.001), and sonography (OR = 18.84; 95% CI 6.40 to 53.33; P < 0.001) than others. Participants with low and moderate breast cancer perception scores were more likely to perform BSE than women with high breast cancer perception scores (OR = 2.20; 95% CI 1.21 to 4.00; P = 0.009) and women who had a history of benign breast disease were more likely to perform screening behaviors than others (OR = 2.47; 95% CI 1.27 to 4.80; P = 0.008). Women between the ages of 50 and 59 were more likely to undergo mammography (OR = 2.33; 95% CI 1.29 to 4.77; P = 0.008) and CBE (OR = 2.40; 95% CI 1.347 to 4.20; P = 0.003) than those ≥ 60 years. Given the low participation of women in regular breast cancer screening, it is suggested that health care providers highlight the need for screening at the specified intervals in their training programs. In addition, health authorities are recommended to use reminder systems to remind women, especially those over 40 years of age, of the best time for breast screening. Moreover, health care providers must seek to improve breast cancer knowledge, attitudes, and perceptions of women who visit health centers, which are the first level of contact with the healthcare system for the general population.
Similar content being viewed by others

Metabolically healthy obesity: from epidemiology and mechanisms to clinical implications

Effects of a personalized nutrition program on cardiometabolic health: a randomized controlled trial
The psychological drivers of misinformation belief and its resistance to correction
Introduction.
Breast cancer is one of the most serious health conditions worldwide, and the most common malignancy in women 1 . It is estimated that by the end of 2024, 310,720 new cases of breast cancer will be added to the number of women diagnosed with breast cancer in the United States, and 42,250 deaths will occur due to this disease 2 . In 2021, about 1.7 million new cases of breast cancer were identified worldwide, accounting for about 25% of all cancer cases in women 3 .
According to world health organization (WHO) reports, the number of breast cancer cases in the Middle East is expected to double by 2030 4 . In a 2017 National Cancer Registry Program, breast cancer was cited as the most common cancer in Iran and East Azerbaijan Province. In addition, the age-standardized incidence rate of breast cancer in Iran and East Azerbaijan was reported to be 43.02 and 40.72 (per 100,000 people), respectively 5 . In Iran, breast cancer occurs mainly in women between the ages of 45 and 55, indicating that Iranian women develop breast cancer a decade earlier than those in developed countries 6 . Therefore, breast cancer is considered a serious, life-threatening disease in women 7 .
Breast cancer is among the most preventable cancers 8 ; lifestyle changes and early diagnosis can reduce the incidence and mortality rates of this cancer 9 . Primary prevention of cancer involves identifying relevant causative and risk factors and offering solutions to reduce these factors. Secondary prevention, on the other hand, includes timely screening and rapid treatment of cancer patients 10 . According to the guidelines of the Iranian Ministry of Health and Medical Education, there are three main methods for breast cancer screening, women over 20 years are recommended to perform breast self-examinations (BSE) monthly, while clinical breast examinations (CBE) by a healthcare professional are recommended annually for those over 40 years. Imaging techniques like mammograms and ultrasounds are also generally advised annually for women aged over 40 11 . A woman can perform BSE at any time and place monthly at no cost. The American Cancer Society (ACS) recommends that women over 40 years of age undergo mammography and CBE every year. In addition, women are recommended to have CBEs every 3 years between the ages of 20 and 40. Both mammography and CBE reduce breast cancer mortality rates by facilitating early detection and treatment 12 . Key goals of the Healthy People Initiative 2020–2030, launched by the U.S. Office of Disease Prevention and Health Promotion, include reducing breast cancer deaths, decreasing the number of late-stage breast cancer patients, and enhancing diagnostic behavior 7 .
Factors such as sociodemographic characteristics (e.g., age, educational qualifications, and income status), beliefs, and attitudes can affect women’s screening behavior 13 . Behavioral models and theories such as the Health Belief Model (HBM) highlight the crucial role of beliefs and cognitions in inspiring people to engage in healthy or risky behaviors (e.g., following or rejecting screening recommendations of physicians) 14 . According to the HBM, identifying patients’ negative beliefs and attitudes would help improve the effectiveness of training and treatments offered by health care providers 15 . Due to their poor health literacy, many women are unaware of the importance of cancer screening 16 . Health literacy refers to a person’s ability to receive, process, perceive, and understand health-related information in order to make appropriate health decisions. It is also an important factor that empowers women to take preventive measures to promote the health of themselves and their children 17 . Research suggests that inadequate health literacy has negative consequences, particularly for cancer control, such as poor understanding of cancer risks, low perception of the importance of screening, and poor participation in preventing adverse clinical outcomes 18 . Low health literacy is generally associated with poor knowledge of cancer screening, unwillingness to undergo cancer examinations, limited access to treatment, improper use of medications, non-adherence to physician recommendations, an increase in hospitalization rates, and a heavy financial burden on the individual, family, and society 19 .
Considering the high prevalence of breast cancer in Iran and worldwide, its good prognosis, the tremendous importance of early diagnosis and screening, and the lack of studies on breast cancer screening patterns and related factors in Iran, this study investigated the screening patterns and associated factors in women over 40 years of age visiting health centers in Tabriz, Iran.
Study design and participants
In this descriptive-analytical cross-sectional study, 372 women over 40 years of age visiting health centers in Tabriz to receive various health services in 2022–2023 participated. Tabriz is one of the largest cities in northwestern Iran and the capital city of East Azerbaijan Province. With a population of over 1.7 million people, Tabriz has 10 municipal districts and 83 health centers.
The study included women over 40 years, regardless of marital status, who regularly visited health centers. Women with major, documented mental health conditions (such as major depressive disorder, bipolar disorder, Schizophrenia, or any other mental illness requiring ongoing medical treatment) in the SIB System or a history of breast cancer were excluded. The initial sample size was calculated as 171 based on the study of Taylan et al. 15 , considering the largest standard deviation of the domain of perceived fear, SD = 1.09, α = 0.05, d = 0.05, and mean = 3.27. The final sample size was determined as 342, taking into account a design effect of 2. However, because a large number of women visited the centers and the sampling process was very easy, 372 women were finally included in the study.
Participants were selected between November 2022 and March 2023 using cluster sampling. To this end, the researcher first randomly selected two health centers from each of the ten districts of Tabriz. After obtaining the list of eligible women from the “SIB System”, participants from each center were selected using proportional allocation and random numbers available on the “ www.random.org ” website. Then, the researcher screened the selected women by telephone for inclusion and exclusion criteria and briefly informed the eligible ones of the research objectives. The women were then asked to visit the respective health centers at a specific time to participate in the study. In the next step, an in-person session was held to explain the research objectives to all participants and to obtain informed consent from those who were willing to take part in the study. Finally, the researcher interviewed the participants and completed the research questionnaires.
Data collection tools
The Sociodemographic Characteristics Questionnaire (SCQ), the Breast Cancer Perception Scale (BCPS), the Health Literacy for Iranian Adults (HELIA) Scale, and the Breast Cancer Screening Behavior Checklist were used to collect the data.
SCQ consisted of 15 items, including age, employment status, marital status, spouse’s job, spouse’s age, spouse’s education, and income sufficiency (the level of income that allows an individual or household to fully meet their basic needs and having acceptable standard of living), number of children, history of underlying diseases, history of breast cancer in family members, long-term use of hormonal medications (e.g., birth control pills), menopausal status, and self- or family history of benign breast disease.
BCPS was designed by Taylan et al. (2021) based on the HBM and its psychometric properties was assessed. The six domains of this 24-item scale include perceived knowledge (items 1–4), perceived treatment belief (items 5–9), perceived need for a health check (items 10–13), perceived stigma (items 14–17), perceived fear (items 18–21), and perceived risk (items 22–24). The items are scored on a five-point Likert scale from strongly disagree (1) to strongly agree (5), but items 9, 10, 11, 12, and 13 are scored inversely. Score range was between 24 and 120 and higher total scores indicate greater perception of breast cancer. Taylan et al. confirmed the construct and content validity of the instrument, and the reliability of all its domains was confirmed with Cronbach’s alpha values ranging from 0.81 to 0.95 15 .
The Health Literacy for Iranian Adults (HELIA) Scale developed by Montazeri et al. (2014) was used to measure the level of health literacy. The five subscales of this 33-item tool include reading (4 items), understanding (7 items), access (6 items), decision (12 items), and appraisal (4 items). A five-point Likert scale ranging from never (score 1) to always (score 5) is used to score the items. However, the items in the reading subscale are scored on a five-point Likert scale ranging from extremely difficult (score 1) to extremely easy (score 5). Score range was between 33 and 165 and higher score indicates higher health literacy. Montazeri et al. confirmed HELIA’s internal consistency reliability with a Cronbach’s alpha value of 0.88 and verified its content validity with a content validity index (CVI) and a content validity ratio (CVR) of 0.79 and 0.85, respectively in Iranian population 20 .In this study, the validity of HELIA scale was assessed only qualitatively using a survey of 10 faculty members of the Tabriz University Medical Sciences, and the scale items were not changed. We assessed the internal consistency of HELIA scale using Cronbach's alpha coefficient, which resulted in a value of 0.95.
The researcher designed a self-report 4-item checklist based on the guidelines of the Iranian Ministry of Health and Medical Education to examine participants’ breast cancer screening behaviors. This checklist was used by trained research staff to verbally assess participants' attendance at screening programs. The Persian version of this checklist is available as supplementary file.
Before starting the study, the face and content validity of the BCPS and the Breast Cancer Screening Behavior Checklist were assessed using quantitative and qualitative methods. In the qualitative phase, 10 faculty members of Tabriz University of Medical Sciences reviewed the Persian version of the questionnaires and provided corrective feedbacks on the use of correct vocabulary, grammar, etc. In the quantitative phase, CVR and CVI values were calculated. Based on Lawshe table, the minimum acceptable CVI and CVR values are 0.79 and 0.62, respectively. The CVI and CVR values for the BCPS were 0.98 and 0.95, respectively, whereas the values for the Breast Cancer Screening Behavior Checklist were 0.87 and 0.84, respectively. The reliability of BCPS and breast cancer screening behaviors checklist was assessed using the test–retest reliability. The intra-class correlation coefficient (ICC) for 30 individuals who completed the questionnaires twice at a two-week interval was calculated to be 0.97 for BCPS and 0.81 for breast cancer screening behaviors checklist. In addition, Cronbach’s alpha values of 0.68 was obtained for BCPS.
Data analysis
The data were analyzed by SPSS version16. The normality of the quantitative data was first confirmed by assessing skewness and kurtosis and visual charts. Then, descriptive statistics of frequency (percentage) and mean (SD) were used to examine participants’ breast cancer perception, screening behavior, and health literacy levels. The binary logistic regression test was used to examine the relationship between screening behaviors and other research variables. Accordingly, multivariate logistic regression analysis was performed with backward strategy to adjust the socio-demographic variables. Here, each of the screening behaviors (BSE, CBE, mammography, and sonography) was considered separately as a dependent variable, and variables that had significant relationships with each of these variables ( p < 0.2) were inserted as independent variables into a backward stepwise multivariate logistic regression analysis model. P value < 0.05 was considered significant.
Ethics approval and consent to participate
The current study received approval from the Research Vice-Chancellor and the Ethics Committee at Tabriz University of Medical Sciences under the code IR.TBZMED.REC.1401.704. Initially, the objectives of the research, participant anonymity, voluntary involvement, and study details were verbally communicated. Subsequently, these were read and acknowledged through a signed written informed consent form. The research methodology adhered to the principles of the Helsinki Declaration.
The study sample consisted of 372 eligible women over 40 years of age visiting health centers in Tabriz. Abou half of participants (54.6%) were housewives. Most of them (79.6%) were married, and had sufficient income (covering expenses or more) (60.7%). In addition, 39 individuals (10.5%) and 74 individuals (19.9%) had a personal history and a family history of benign breast disease, respectively. Table 1 shows other sociodemographic characteristics of participants.
Overall, 68.3% of all women had performed BSE at least once, but only 9.9% of them performed regular monthly examinations. In addition, 60.2% of women underwent CBE at least once, but only 8.9% of them underwent regular CBE every 6 months. Moreover, 51.3% of participants underwent mammography at least once, but only 12.3% of them attended regular annual mammography sessions. Finally, 38.2% of women underwent sonography at least once, but only 3.8% of them had regular sonography every 6 months (Table 2 ).
The univariate logistic regression analysis results showed that variables of age, educational qualifications, spouse’s age, spouse’s educational qualifications, spouse’s job, history of underlying diseases, history of breast cancer in family members, self or family history of benign breast disease, breast cancer perception, and health literacy had significant relationships with BSE ( p < 0.2). Therefore, these variables were entered into a backward stepwise multivariate analysis model. The results showed that both family history of benign breast disease (OR = 2.47; 95% CI 1.27 to 4.80; P = 0.008) and breast cancer perception (OR = 2.20; 95% CI 1.21 to 4.00; P = 0.009) were significantly associated with BSE. In other words, women with a family history of benign breast disease were 2.47 times more likely to perform BSE than others. In addition, women with low and moderate breast cancer perception scores were 2.2 times more likely to perform BSE than those with high perception scores (Table 3 ).
The univariate logistic regression analysis results showed that variables of age, number of children, spouse’s age, self- or family history of benign breast disease, breast cancer perception, and health literacy had significant relationships with CBE ( p < 0.2). Therefore, these variables were entered into a backward stepwise multivariate analysis model. Based on the results, variables of age (OR = 2.40; 95% CI 1.347 to 4.20; P = 0.003) and personal history of benign breast disease (OR = 8.49; 95% CI 2.55 to 28.21; P < 0.001) were significantly related to CBE. In other words, women who had a history of benign breast disease were 8 times more likely to undergo CBE than others. In addition, women between the ages of 50 and 59 were 2.4 times more likely to undergo CBE than those over 60 years (Table 3 ).
The univariate logistic regression analysis results showed that variables of age, employment status, spouse’s age, income status, history of underlying diseases, history of breast cancer in family members, history of use of hormonal medications, self or family history of benign breast disease, and breast cancer perception had significant relationships with mammography screening ( p < 0.2). These variables were entered into a backward stepwise multivariate analysis model. The results indicated that variables of age (OR = 2.33; 95% CI 1.29 to 4.77; P = 0.008) and personal history of benign breast disease (OR = 8.84; 95% CI 2.98 to 10; P < 0.001) were significantly associated with mammography screening. This implied that women who had a history of benign breast disease were 8.8 times more likely to undergo mammography than others. In addition, women between the ages of 50 and 59 were 2.38 times more likely to undergo mammography than those over 60 years (Table 3 ).
Finally, the univariate logistic regression analysis results showed that variables of educational qualifications, spouse’s age, spouse’s educational qualifications, number of children, income status, history of underlying diseases, history of breast cancer in family members, self- or family history of benign breast disease, and breast cancer perception had significant relationships with sonography screening ( p < 0.2). After inserting these variables into a backward stepwise multivariate analysis model, the results showed that variable of personal history of benign breast disease (OR = 18.84; 95% CI 6.40 to 53.33; P < 0.001) was significantly associated with sonography screening. In other words, women who had a history of benign breast disease were 18.48 times more likely to undergo sonography than others (Table 3 ).
This study aimed to determine the breast cancer screening behaviors patterns and associated factors among women over 40 years of age. Based on the findings, more than half of participants experienced BSE, CBE, and mammography at least once; however, few performed these examinations regularly and according to recommended guidelines. A history of benign breast disease was significantly associated with all four screening behaviors, as women who had a history of benign breast disease were more likely to perform screening behaviors than others. Women with low and moderate breast cancer perception scores were more likely to perform BSE than those with high breast cancer perception scores. In addition, women between the ages of 50 and 59 were more likely to undergo mammography and CBE than those ≥ 60 years.
Our study found that while 68.3% of participants reported performing BSE at least once, only 9.9% performed it regularly each month. This highlights a gap in adherence, similar to findings from other studies. For instance, Kwok (2020) observed that in Korean-Australian women, despite awareness of breast cancer screening methods (including BSE), only 31.4% performed regular BSE 21 . Similarly, a study in Korea reported high awareness of BSE benefits (88%) but low practice (29.3%), with many participants citing lack of proper knowledge (31.7%) as a barrier 22 . Likewise, another study in Istanbul, Turkey, found only 32.1% of women performing regular BSE 23 .
The prevalence of regular BSE in this study was about 10% which is lower than in similar studies. Unfamiliarity with recommended intervals might be a contributing factor. Healthcare providers should emphasize the importance of regular BSE practice. Currently, there is continuous debate about the effectiveness of BSE and CBE in reducing mortality rates. As a result, some international organizations no longer recommend these examinations as screening measures for detecting breast cancer. However, in less developed countries, where women are often diagnosed with breast cancer at a younger age and at advanced stages, the advantages of these methods may outweigh their disadvantages and facilitate early detection of breast cancer 24 . The results of a recent 5 year follow-up study showed that women who perform BSE irregularly have a 1.31 times higher risk of developing late-stage breast cancer and a 1.70 times higher risk of dying from breast cancer than those who perform it regularly. In addition, women who had regular BSE screening had significantly smaller tumors, earlier cancer stage, and higher survival rates than others. In developing countries, women between the ages of 50 and 74 are usually recommended to have regular mammograms every 2 or 3 years. However, since mammography services are not extensively provided to younger women, BSE is still widely used in these countries 25 .
Similar to BSE, CBE adherence was low in our study, with only 8.9% of women receiving regular screenings. In a similar study in Iran by Rabiei et al. (2022), it was reported a 52.6% prevalence of ever-performed CBE in Iranian women, but did not specify regular screening rates 26 . The prevalence of CBE in this study (60.2%) was higher than in the study of Rabiei et al. (52.6%). A study in Malaysia in 2010 also found a lower prevalence (25%) of ever-performed CBE among female teachers 27 . The difference is probably due to the older age of the participants in this study compared to similar studies. Accordingly, recent studies have found a direct association between age and the prevalence of CBE. CBE performed by health care providers at recommended intervals significantly lowers the stage of cancer diagnosis and reduces mortality by 15% in women. CBE also results in a 30% reduction in mortality rates in women older than 50 years 28 . In contrast, the findings of a study on 11 systematic reviews showed no direct evidence that CBE reduces mortality in breast cancer patients; however, CBE reduced the likelihood of shifting from early-stage to advanced-stage cancer by 17–47% 29 .
This study found that while over half (51.3%) of participants underwent mammography at least once, only 12.3% adhered to recommended screening intervals. This low adherence rate aligns with findings from developing countries 21 , 30 , 31 , where prevalence of screening is generally lower compared to developed nations (e.g., 70% in the United States) 32 . This suggests that interventions beyond awareness campaigns might be needed in developing countries. Furthermore, research in developed nations, such as the United States 32 , has identified physician recommendation as a key factor influencing screening behavior. This suggests a valuable direction for future research in developing countries. Regarding that in the present study only a small percentage of women had mammograms at the recommended intervals, regularly visits for older women by doctors at the first level of the healthcare system and advising them to have regular mammograms is suggested.
Self or family history of benign breast disease was significantly associated with all four screening behaviors. In line with this finding, studies report that a family history of cancer 33 , 34 and a history of breast diseases in oneself 35 , friends, and peers 36 have significant positive relationships with breast cancer screening behaviors such as CBE and mammography. In addition, perceived cancer risk is higher in these women than in others, and according to the HBM, high perceived risk is related to a high likelihood of engaging in preventive behaviors. In a recent study, 70% of participants reported family history as the main risk factor for developing breast cancer. For this reason, women with a family history of benign breast disease are more inclined than others to participate in breast cancer screening 37 . In this study, women with a family history of benign breast disease were more likely to perform BSE than others. In contrast to the present results, Guo observed no consistent relationship between family history of breast lumps and screening behavior (mammography and CBE) 38 . The discrepancy between Guo’s findings and the present results can be attributed to the fact that some women experience psychological problems such as fear, and therefore exhibit avoidance behaviors. Regardless of personal or family history, regular mammograms remain crucial for all women within the recommended age range. Therefore, future public health initiatives should prioritize messaging that underscores the importance of regular screening for all women within the recommended age range, irrespective of perceived risk.
This study found a positive association between age and both CBE and mammography utilization. This aligns with some existing research, where older women were reported to be more likely to undergo screening 36 . However, conflicting evidence also exists 39 . Consistent with the findings of this study, a 2018 systematic review in Malaysia reported age as an important predictor of mammography because older women were more likely to seek mammography 40 . These discrepancies might be due to factors like cost and insurance coverage, as suggested in studies where younger women had limited access to mammograms due to insurance policies 39 . For example, some health insurance companies may not cover screening mammograms for women younger than 40 years 41 . This highlights the potential influence of socioeconomic factors on screening behavior. It's important to note that some studies report a decrease in screening adherence among women over 65 years old 42 . This suggests a more nuanced relationship between age and screening behavior, where factors like health status might play a role in later life. Additionally, research suggests that interventions like increasing awareness campaigns, reminder systems, and offering subsidized or free screening services, particularly for older women, can be effective in improving screening rates 43 .
This study found an intriguing relationship between breast cancer perception and BSE behavior. Women with lower and moderate perceptions were more likely to perform BSE compared to those with high perception. While the reasons for this require further investigation, existing research suggests a connection between breast cancer awareness, knowledge, and health-seeking behaviors 44 . It's possible that women with lower perceptions might have low perceived need for a health check, poor perceived treatment beliefs, and great perceived fear of breast cancer leading them to rely on self-examinations (BSE) as a form of control or reassurance. Rainey et al. (2019) examined women’s perceptions of breast cancer screening in three countries and concluded that women’s perceptions of screening, which are rooted in behavioral theory, are influenced by factors such as lack of knowledge, cultural norms, and common emotional concerns 45 . Therefore, providing appropriate educational materials and risk counseling programs can help women make informed collective or individual decisions. It should be noted that acceptance of risk-based screening and prevention of breast cancer are intertwined 21 . Accordingly, appropriate educational programs should be provided at the national level to institutionalize screening behaviors among women by improving their perception and raising their awareness of breast cancer. Nurses can play a vital role in raising breast cancer awareness among women by organizing comprehensive screening programs 46 .
Strengths and limitations
In this study, the factors associated with each of the four recommended screening behaviors were examined separately that can consider as a strength of our study. The second strength of the study was the random selection of participants among all health centers in Tabriz. However, our study has some limitations. Use of self-report questionnaires increased the risk of bias in this study. Moreover, due to the cross-sectional nature of the study, the relationships among the research variables cannot be considered as cause-and-effect relationships. Our reliance on a selected cohort of consenting women limits the generalizability of the findings to the broader Iranian population. A broader retrospective analysis across the healthcare system could mitigate this limitation.
Given the low participation of women in regular breast cancer screening, it is suggested that health care providers emphasize the need for screening at the specified intervals in their training programs. Considering the high prevalence of breast cancer in Iran, relevant health authorities are recommended to use reminder systems to remind Iranian women, especially those over 40 years of age, of the best time for breast screening. Moreover, health care providers must seek to improve breast cancer knowledge, attitudes, and perceptions of women who visit health centers, which are the first level of contact with the healthcare system for the general population.
Data availability
The datasets used and/or analyzed in the current study are available through the corresponding author for scientific use, such as replication.
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68 (6), 394–424 (2018).
Article PubMed Google Scholar
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics. CA Cancer J. Clin. 74 (1), 12–49 (2024).
Arnold, M. et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 66 , 15–23 (2022).
Article PubMed PubMed Central Google Scholar
World Health Organization Eastern Mediterranean Region (WHO EMRO). (n.d.). Cancer control|Highlights. https://www.emro.who.int/noncommunicable-diseases/publications/cancer-control.html .
Haghighat, S., Omidi, Z. & Ghanbari-Motlagh, A. Trend of breast cancer incidence in Iran during a fifteen-year interval according to national cancer registry reports. Iran. J. Breast Dis. 15 (2), 4–17 (2022).
Article Google Scholar
Ansari, H., Haji-Gholami, A. & Moradi-Vastegani, Z. Personality traits score in women with breast cancer and its comparison with healthy women. J. Isfahan Med. School 39 (622), 277–283 (2021).
Google Scholar
Centers for Disease Control and Prevention (CDC). (2010). Healthy people 2020. https://www.cdc.gov/nchs/healthy_people/hp2020.htm .
Harandy, T. F. et al. Health-related quality of life in iranian breast cancer survivors: A qualitative study. Payesh (Health Monitor) 11 (1), 73–81 (2012).
Mahdavifar, N. et al. Spatial analysis of breast cancer incidence in Iran. Asian Pac. J. Cancer Prevent. 17 , 59–64 (2016).
Mohaghegh, P., Farahani, M., Moslemi, A., Ahmadi, F. & Nazari, J. Participation rate, family histories, symptoms, and incidence of breast cancer in the screening program for breast cancer in the population covered by arak health centers. Iran. J. Breast Dis. 14 (2), 41 (2021).
Screening behaviour of breast cancer 1401 Available from: https://behdasht.gov.ir/ .
Seely, J. & Alhassan, T. Screening for breast cancer in 2018—what should we be doing today?. Curr. Oncol. 25 (s1), 115–124 (2018).
Ghofranipour, F., Pourhaji, F. & Delshad, M. H. Determinants of breast cancer screening: Application of protection motivation theory. Int. J. Cancer Manage. 13 (5), 1–7 (2020).
Tan, M. M., Jamil, A. S. A., Ismail, R., Donnelly, M. & Su, T. T. Breast cancer and breast cancer screening use—beliefs and behaviours in a nationwide study in Malaysia. BMC Public Health 23 (1), 1319 (2023).
Taylan, S., Özkan, İ & Adıbelli, D. Breast cancer perception scale: Psychometric development study. Eur. J. Breast Health 17 (2), 95 (2021).
Ghanbari, A., Rahmatpour, P., Khalili, M. & Mokhtari, N. Health literacy and its relationship with cancer screening behaviors among the employees of Guilan university of medical sciences. J. Health Care 18 (4), 306–315 (2017).
Badr, H. & Shen, M. J. Pain catastrophizing, pain intensity, and dyadic adjustment influence patient and partner depression in metastatic breast cancer. Clin. J. Pain 30 (11), 923 (2014).
Rakhshkhorshid, M., Navaee, M., Nouri, N. & Safarzaii, F. The association of health literacy with breast cancer knowledge, perception and screening behavior. Eur. J. Breast Health 14 (3), 144 (2018).
PubMed PubMed Central Google Scholar
Coughlin, S. et al. Health literacy, social determinants of health, and disease prevention and control. J. Environ. Health Sci. 6 (1), 3061 (2020).
Montazeri, A. et al. Health literacy for Iranian adults (HELIA): Development and psychometric properties. Inf. J Pap. 13 , 589 (2014).
Kwok, C., Lee, M.-J. & Lee, C. F. Breast cancer perceptions and screening behaviours among Korean women in Australia. J. Immigrant Minor. Health 22 , 126–133 (2020).
Yoo, B. N., Choi, K. S., Jung, K. W. & Jun, J. K. Awareness and practice of breast self-examination among Korean women: Results from a nationwide survey. Asian Pac. J. Cancer Prev. 13 (1), 123–125 (2012).
Gür, K., Kadıoğlu, H. & Sezer, A. Breast cancer risks and effectiveness of BSE training among women living in a district of Istanbul. J. Breast Health 10 (3), 154–160 (2014).
Albeshan, S. M., Hossain, S. Z., Mackey, M. G. & Brennan, P. C. Can breast self-examination and clinical breast examination along with increasing breast awareness facilitate earlier detection of breast cancer in populations with advanced stages at diagnosis?. Clin. Breast Cancer 20 (3), 194–200 (2020).
Thaineua, V. et al. Impact of regular breast self-examination on breast cancer size, stage, and mortality in Thailand. Breast J. 26 (4), 822–824 (2020).
Rabiei, M., Hoseini, S. H., Khodarahmi, S., Sepahvand, E. & Shirali, E. Factors related to clinical breast examination: A cross-sectional study. J. Fam. Med. Prim. Care 11 (6), 3051 (2022).
Parsa, P. & Kandiah, M. Predictors of adherence to clinical breast examination and mammography screening among Malaysian women. Asian Pac. J. Cancer Prev. 11 (3), 681–688 (2010).
PubMed Google Scholar
Mittra, I. et al. Effect of screening by clinical breast examination on breast cancer incidence and mortality after 20 years: Prospective, cluster randomised controlled trial in Mumbai. BMJ 372 , n256 (2021).
Ngan, T. T., Nguyen, N. T. Q., Van Minh, H., Donnelly, M. & O’Neill, C. Effectiveness of clinical breast examination as a “stand-alone” screening modality: An overview of systematic reviews. BMC Cancer 20 (1), 1070 (2020).
Tarı Selçuk, K., Avcı, D., Yılmaz Dündar, G. & Mercan, Y. Breast cancer screening behaviors in women aged 40 years and over in a semi-urban region in Turkey: Relationships with health beliefs. Healthcare 8 , 171 (2020).
Zahedi, R. et al. Breast cancer risk perception and screening behaviors of Iranian women. Women’s Health Bull. 8 (2), 98–106 (2021).
Cohen, S. S., Signorello, L. B., Gammon, M. D. & Blot, W. J. Obesity and recent mammography use among black and white women in the southern community cohort study (United States). Cancer Causes Control 18 , 765–773 (2007).
Abolfotouh, M. A. et al. Using the health belief model to predict breast self examination among Saudi women. BMC Public Health 15 , 1–12 (2015).
Dewi, T. K., Massar, K., Ruiter, R. A. & Leonardi, T. Determinants of breast self-examination practice among women in Surabaya, Indonesia: An application of the health belief model. BMC Public Health 19 (1), 1–8 (2019).
Wu, Z. et al. Factors associated with breast cancer screening participation among women in mainland China: A systematic review. BMJ Open 9 (8), e028705 (2019).
Alduraibi, S. K. Breast cancer knowledge and screening behaviors of female teachers: A cross-sectional survey in Buraydah, Saudi Arabia. J. Fam. Med. Prim. Care 11 (7), 3834 (2022).
Beidler, L. B. et al. Perceptions of breast cancer risks among women receiving Mammograph screening. JAMA Netw. Open 6 (1), e2252209 (2023).
Guo, S. Cross-sectional study on knowledge-attitude-practice of breast cancer prevention among community women in Urumqi. The Master’s Thesis. Urumqi: Xinjiang Medical University. (2011).
Wu, T.-Y. & Lee, J. Promoting breast cancer awareness and screening practices for early detection in low-resource settings. Eur. J. Breast Health 15 (1), 18 (2019).
Aidalina, M. & ASJ, S. M. The uptake of mammogram screening in Malaysia and its associated factors: A systematic review. Med. J. Malays. 73 (4), 202–211 (2018).
CAS Google Scholar
Young, J. M. et al. Accuracy of perceived breast cancer risk in black and white women with an elevated risk. Ethn. Dis. 32 (2), 81 (2022).
Bynum, J. P., Braunstein, J. B., Sharkey, P., Haddad, K. & Wu, A. W. The influence of health status, age, and race on screening mammography in elderly women. Arch. Intern. Med. 165 (18), 2083–2088 (2005).
Lee, K., Lim, H. T. & Park, S. M. Factors associated with use of breast cancer screening services by women aged≥ 40 years in Korea: The third Korea national health and nutrition examination survey 2005 (KNHANES III). BMC Cancer 10 (1), 1–11 (2010).
Article ADS Google Scholar
Ossai, E. et al. Predictors of practice of breast self-examination: A study among female undergraduates of Ebonyi state university, Abakaliki, Nigeria. Niger. J. Clin. Pract. 22 (3), 361–369 (2019).
Article CAS PubMed Google Scholar
Rainey, L. et al. Women’s perceptions of personalized risk-based breast cancer screening and prevention: An international focus group study. Psycho-Oncology 28 (5), 1056–1062 (2019).
Mahmoud, A. A., Abosree, T. H. & Abd El Aliem, R. S. Effect of the health belief model-based education on preventive behaviors of breast cancer. Evid. -Based Nurs. Res. 2 (4), 11 (2020).
Download references
Acknowledgements
We would like to thank Tabriz University of Medical Sciences for financial support and all women who participated in this study. We sincerely appreciate their support.
This research study was supported by the Tabriz University of Medical Sciences.
Author information
Authors and affiliations.
Department of Community Health Nursing, Nursing and Midwifery Faculty, Tabriz University of Medical Sciences, Tabriz, Iran
Elham Seyedkanani, Mina Hosseinzadeh & Leila Sheikhnezhad
Social Determinants of Health Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Mojgan Mirghafourvand
You can also search for this author in PubMed Google Scholar
Contributions
E.SK. has contributed to the study conception, design, data analysis, manuscript preparation, editing and review. M.H. contributed to the study design, data analysis, manuscript editing and review. MM performed the data analysis, manuscript preparation, editing and review. E.SK. made contributions to the conception, design, acquisition, analysis and interpretation of data and prepared the first draft. L.SH. revised the final draft of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Correspondence to Mina Hosseinzadeh .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Seyedkanani, E., Hosseinzadeh, M., Mirghafourvand, M. et al. Breast cancer screening patterns and associated factors in Iranian women over 40 years. Sci Rep 14 , 15274 (2024). https://doi.org/10.1038/s41598-024-66342-0
Download citation
Received : 10 November 2023
Accepted : 01 July 2024
Published : 03 July 2024
DOI : https://doi.org/10.1038/s41598-024-66342-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Arab women's breast cancer screening practices: a literature review
Affiliation.
- 1 Faculty of Nursing and Medicine, The University of Calgary and the University of Calgary-Qatar, Qatar E-mail : [email protected].
- PMID: 24083695
- DOI: 10.7314/apjcp.2013.14.8.4519
Breast cancer incidence and mortality rates are increasing in the Arab world and the involved women are often diagnosed at advanced stages of breast cancer. This literature review explores factors influencing Arab women's breast cancer screening behavior. Searched databases were: Medline, PubMed, Cochrane Database of Systematic Reviews, CINAHL Plus, Google Scholar, Index Medicus for WHO Eastern Mediterranean, and Asian Pacific Journal of Cancer Prevention. Breast cancer screening participation rates are low. Screening programs are opportunistic and relatively new to the region. Knowledge amongst women and health care providers, professional recommendation, socio-demographic factors, cultural traditions, beliefs, religious, social support, accessibility and perceived effectiveness of screening influence screening behavior.
PubMed Disclaimer
Similar articles
- Barriers and Facilitators Influencing Arab Muslim Immigrant and Refugee Women's Breast Cancer Screening: A Narrative Review. Racine L, Isik Andsoy I. Racine L, et al. J Transcult Nurs. 2022 Jul;33(4):542-549. doi: 10.1177/10436596221085301. Epub 2022 Apr 26. J Transcult Nurs. 2022. PMID: 35473467 Free PMC article. Review.
- Sociocultural Influences on Arab Women's Participation in Breast Cancer Screening in Qatar. Hwang JJ, Donnelly TT, Ewashen C, McKiel E, Raffin S, Kinch J. Hwang JJ, et al. Qual Health Res. 2017 Apr;27(5):714-726. doi: 10.1177/1049732315619373. Epub 2016 Jul 10. Qual Health Res. 2017. PMID: 26631675
- Factors that influence awareness of breast cancer screening among Arab women in Qatar: results from a cross sectional survey. Donnelly TT, Khater AH, Al-Bader SB, Al Kuwari MG, Malik M, Al-Meer N, Singh R, Fung T. Donnelly TT, et al. Asian Pac J Cancer Prev. 2014;15(23):10157-64. doi: 10.7314/apjcp.2014.15.23.10157. Asian Pac J Cancer Prev. 2014. PMID: 25556441
- Application of health behavior theories to breast cancer screening among Asian women. Ahmadian M, Samah AA. Ahmadian M, et al. Asian Pac J Cancer Prev. 2013;14(7):4005-13. doi: 10.7314/apjcp.2013.14.7.4005. Asian Pac J Cancer Prev. 2013. PMID: 23991945 Review.
- Health beliefs and rates of breast cancer screening among Arab women. Azaiza F, Cohen M. Azaiza F, et al. J Womens Health (Larchmt). 2006 Jun;15(5):520-30. doi: 10.1089/jwh.2006.15.520. J Womens Health (Larchmt). 2006. PMID: 16796479
- Knowledge, Attitudes and Practices of Women in the UAE Towards Breast and Cervical Cancer Prevention: A Cross-Sectional Study. Elbarazi I, Alam Z, Abdullahi AS, Al Alawi S, AlKhanbashi M, Rabaa A, Al Aryani A, Ahmed L, Al-Maskari F. Elbarazi I, et al. Cancer Control. 2023 Jan-Dec;30:10732748231211459. doi: 10.1177/10732748231211459. Cancer Control. 2023. PMID: 37950611 Free PMC article.
- Awareness of and Attitude to Breast Self-Examination and Breast Cancer Among Females in Jeddah, Saudi Arabia. Alqarni GS, Musslem MT, Alosaimi RM, Filfilan FF, Al Qarni AS, Rizk H. Alqarni GS, et al. Cureus. 2023 Mar 23;15(3):e36595. doi: 10.7759/cureus.36595. eCollection 2023 Mar. Cureus. 2023. PMID: 37095809 Free PMC article.
- How Do Healthy Women Perceive the Risk of Breast Cancer? The Role of Illness Perceptions and Compared Risk between Portugal and the U.A.E. Figueiras MJ, Neto DD, Marôco J, Carmo C. Figueiras MJ, et al. Int J Environ Res Public Health. 2022 Oct 9;19(19):12923. doi: 10.3390/ijerph191912923. Int J Environ Res Public Health. 2022. PMID: 36232223 Free PMC article.
- Sexual activity and cancer: A systematic review of prevalence, predictors and information needs among female Arab cancer survivors. Alananzeh I, Green H, Meedya S, Chan A, Chang HCR, Yan Z, Fernandez R. Alananzeh I, et al. Eur J Cancer Care (Engl). 2022 Nov;31(6):e13644. doi: 10.1111/ecc.13644. Epub 2022 Jul 11. Eur J Cancer Care (Engl). 2022. PMID: 35816027 Free PMC article. Review.
- Breast Cancer Knowledge, Attitudes and Practices amongst Women in Qatar. Hamed E, Alemrayat B, Syed MA, Daher-Nashif S, Rasheed HMA, Kane T. Hamed E, et al. Int J Environ Res Public Health. 2022 Mar 28;19(7):3995. doi: 10.3390/ijerph19073995. Int J Environ Res Public Health. 2022. PMID: 35409678 Free PMC article.
Publication types
- Search in MeSH
Related information
- Cited in Books
LinkOut - more resources
Full text sources.
- Asian Pacific Organization for Cancer Prevention
- Genetic Alliance
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Systematic review
- Open access
- Published: 22 May 2023
Characteristics and impact of interventions to support healthcare providers’ compliance with guideline recommendations for breast cancer: a systematic literature review
- Ignacio Ricci-Cabello 1 , 2 , 3 ,
- Darla Carvallo-Castañeda 4 ,
- Adrián Vásquez-Mejía 4 ,
- Pablo Alonso-Coello 3 , 5 ,
- Zuleika Saz-Parkinson 6 ,
- Elena Parmelli 6 ,
- Gian Paolo Morgano 6 ,
- David Rigau 5 ,
- Ivan Solà 3 , 5 ,
- Luciana Neamtiu 6 &
- Ena Niño-de-Guzmán 5 , 7
Implementation Science volume 18 , Article number: 17 ( 2023 ) Cite this article
2838 Accesses
12 Altmetric
Metrics details
Breast cancer clinical practice guidelines (CPGs) offer evidence-based recommendations to improve quality of healthcare for patients. Suboptimal compliance with breast cancer guideline recommendations remains frequent, and has been associated with a decreased survival. The aim of this systematic review was to characterize and determine the impact of available interventions to support healthcare providers’ compliance with CPGs recommendations in breast cancer healthcare.
We searched for systematic reviews and primary studies in PubMed and Embase (from inception to May 2021). We included experimental and observational studies reporting on the use of interventions to support compliance with breast cancer CPGs. Eligibility assessment, data extraction and critical appraisal was conducted by one reviewer, and cross-checked by a second reviewer. Using the same approach, we synthesized the characteristics and the effects of the interventions by type of intervention (according to the EPOC taxonomy), and applied the GRADE framework to assess the certainty of evidence.
We identified 35 primary studies reporting on 24 different interventions. Most frequently described interventions consisted in computerized decision support systems (12 studies); educational interventions (seven), audit and feedback (two), and multifaceted interventions (nine). There is low quality evidence that educational interventions targeted to healthcare professionals may improve compliance with recommendations concerning breast cancer screening, diagnosis and treatment. There is moderate quality evidence that reminder systems for healthcare professionals improve compliance with recommendations concerning breast cancer screening. There is low quality evidence that multifaceted interventions may improve compliance with recommendations concerning breast cancer screening. The effectiveness of the remaining types of interventions identified have not been evaluated with appropriate study designs for such purpose. There is very limited data on the costs of implementing these interventions.
Conclusions
Different types of interventions to support compliance with breast cancer CPGs recommendations are available, and most of them show positive effects. More robust trials are needed to strengthen the available evidence base concerning their efficacy. Gathering data on the costs of implementing the proposed interventions is needed to inform decisions about their widespread implementation.
Trial registration
CRD42018092884 (PROSPERO)
Peer Review reports
Contributions to the literature
Research has shown that compliance with breast cancer clinical practice guidelines remains suboptimal, leading to increased mortality rates.
Our study is the first systematic review evaluating interventions to support compliance with breast cancer clinical practice guidelines recommendations, and builds upon previous reviews of this topic in more general contexts. We found that a number of different types of interventions have been developed and evaluated, most of them showing beneficial effects.
The quality of the evidence is low for provider educational interventions, moderate for provider reminders, and low for multifaceted interventions. For the rest of the interventions identified, the evidence is uncertain.
This review contributes to recognized gaps in the literature, including ascertaining which types of interventions work best to promote compliance with breast cancer CPGs, as well as identifying new areas for future research.
Findings from this review may help those practitioners and health decision makers interested in improving the quality and safety of breast cancer healthcare provision by enhancing the uptake of clinical practice guidelines.
Introduction
Breast cancer is the most common cancer in women with 2.3 million new cases estimated in 2020, accounting for 11.7% of all cancers [ 1 ]. It is the fifth leading cause of cancer mortality worldwide, with 685,000 deaths [ 1 ]. Breast cancer diagnosis is more frequent in developed countries [ 2 ]. Controlling and preventing breast cancer is an important priority for health policy makers [ 3 ].
Treatment procedures have rapidly evolved over recent years. As new and precise diagnosis strategies emerged, early treatment and prognosis of breast cancer patients have shown great progresses [ 4 ]. Advances in breast cancer screening and treatment have reduced the mortality of breast cancer across the age spectrum in the past decade [ 5 , 6 , 7 ]. Although the use of research evidence can improve professional practice and patient-important outcomes, considering also the huge volume of research evidence available, its translation into daily care routines is generally poor [ 8 , 9 ]. It is estimated that it takes an average of 17 years for only 14% of new scientific discoveries to enter day-to-day clinical practice [ 10 ].
Clinical Practice Guidelines (CPGs) provide recommendations for delivering high quality healthcare [ 11 , 12 ]. However, the impact of CPGs depends not only on their quality, but also on the way and the extent to which they are used by clinicians in routine clinical practice. Large overviews show that approximately 50% of patients receive from general medical practitioners treatments which differ from recommended best practice [ 13 , 14 , 15 , 16 ]. In the area of breast cancer, previous systematic reviews have shown that compliance with breast cancer CPGs [ 17 ], as well as for other types of cancer [ 18 , 19 , 20 ], remains suboptimal. A recent systematic review from our research group [ 21 ] found large variations in providers´ compliance with breast cancer CPGs, with adherence rates ranging from 0 to 84.3%. Sustainable use of CPGs is also notably poor: after 1 year of their implementation, adherence decreases in approximately half of the cases [ 22 ].
Suboptimal compliance with CPGs recommendations could increase healthcare costs if healthcare resources are overused (e.g., overtreatment, overuse of diagnosis or of screening techniques); but also, if they are underused (i.e., increased costs to cover the additional health care needs that people may face with worsening conditions due to under-used resources). Available evidence suggests that outcomes may improve for patients, healthcare professionals and healthcare organizations if decision-makers adhere to evidence-based CPGs [ 23 , 24 ]. This is supported by a recent meta-analysis from our group [ 25 ], which suggests that compliance with CPGs is probably associated with an increase in both, disease-free survival (hazard ratio (HR) = 0.35 (95% CI from 0.15 to 0.82)) and overall survival (HR = 0.67 (95% CI 0.59 to 0.76). Developing interventions to support clinician uptake of breast cancer CPGs is therefore essential for improving healthcare quality and patient important outcomes. Although several interventions to support compliance with breast cancer CPGs have been proposed, no previous study has systematically examined their characteristics and effects.
The aim of this systematic review is to characterize and evaluate the impact of available interventions to support healthcare providers’ compliance with CPGs in breast cancer care.
We conducted a systematic literature review adhering to the PRISMA reporting guidelines [ 26 ] (PRISMA 2020 Checklist available at Additional file 1 ). In this review, we addressed the following two questions: (1) What type of interventions have been used to support healthcare professionals´ compliance with breast cancer CPGs? and; (2) What type of interventions can effectively support healthcare professionals’ compliance with breast cancer CPGs? We registered the protocol in the international prospective register of systematic reviews (PROSPERO registration number CRD42018092884).
We searched for systematic reviews and original studies in MEDLINE (through PubMed) and Embase (through Ovid) using predefined search strategies from inception to May 2021 designed and implemented by an information specialist (IS) from the Iberoamerican Cochrane Centre (IS). The search strategies (available in Additional file 2 ) combined MeSH terms and keywords.
Study selection
We applied the following inclusion criteria:
Population: healthcare professionals providing health services related to the prevention or management of breast cancer. All types of healthcare professionals, and from any setting were included.
Intervention: interventions explicitly aimed at supporting or promoting healthcare professionals’ compliance with available breast cancer CPGs. Such guidelines may address any specific aspect of breast cancer care, including screening, diagnosis, treatment, surveillance or rehabilitation.
Comparator: any comparator, including also studies not using a comparator group.
Outcome: quality of breast cancer care (based on healthcare professionals’ compliance rate with breast cancer CPGs recommendations, but also on their knowledge, attitudes or self-efficacy concerning such recommendations); intervention implementation (fidelity, reach, implementation costs), and; patient health-related outcomes (e.g., survival).
We included experimental (randomized controlled and non-randomized controlled trials), observational (before-after, cohort, case-control, cross-sectional, and case studies), and qualitative or mixed-methods studies. Due to constrained resources, we only included studies published in English. One author (of IRC, DC, APVM) screened the search results based on title and abstract. A second author (ENG, LN, ZSP, EP, DC, APVM, GPM) independently reviewed 20% of all references. Two authors independently assessed eligibility based on the full text of the relevant articles. Disagreements were discussed (involving a third author when needed) until consensus was reached.
Data extraction
One author (ENG, IRC LN, ZSP, EP, DC, APVM, GPM) extracted the following data about the characteristics and results of the included studies using an ad hoc data extraction form which had been piloted in advance: publication year, study design (e.g., randomized controlled trial), study location, setting, number of participants, aim of the study, type of breast cancer guideline (e.g., breast cancer screening), type of intervention (e.g., computerized decision support systems), and outcome(s) assessed (e.g., compliance rate). A second author (ENG, IRC LN, ZSP, EP, DC, APVM, GPM) cross-checked the extracted data for accuracy.
Quality assessment
We used the following tools to determine the risk of bias of the included studies: the Cochrane Collaboration tool for assessing risk of bias in randomized trials (RoB I) [ 27 ], the ROBINS I tool for non-randomized controlled before-after studies [ 28 ], the Quality Assessment Tool for Before-After (Pre-Post) Studies With No Control Group [ 29 ], the Newcastle-Ottawa scale for cohort studies [ 30 ], the AXIS tool for cross-sectional studies [ 31 ], and the MMAT tool [ 32 ] for mixed methods studies. The specific criteria included by each of these tools are available in Additional file 3 . One author determined the risk of bias of the included studies, and a second author cross-checked the results for accuracy. Disagreements were solved with support from a senior systematic reviewer.
Data synthesis
We described the characteristics and the effects of the interventions narratively and as tabulated summaries. Findings are synthesized by type of intervention. We applied the Cochrane Effective Practice and Organization Care Review Group (EPOC) [ 33 ] taxonomy to classify our findings according to the types of interventions identified. Whereas for the characterization of the interventions we included all the publications identified meeting our eligibility criteria (irrespectively of their design); for the evaluation of the effectiveness of the interventions we focused only on those studies following a suitable design for such purpose [ 34 ]: randomized controlled trials (RCTs), controlled before-after studies, non-randomized controlled trials, and interrupted time series. Although we planned to conduct a meta-analysis on the impact of the interventions on compliance rates, this was finally not feasible due to the inconsistent and poor reporting. Instead, we provide a graphical quantitative description of the compliance rates before and after the implementation of the interventions.
Certainty of the evidence
Following the GRADE approach [ 35 ], we rated the certainty of evidence as high, moderate, low or very low, taking into consideration risk of bias, imprecision, inconsistency, indirectness, and publication bias. This was done by one researcher. and cross-checked by a second reviewer.
Search results
The eligibility process is summarized in a PRISMA flowchart (Fig. 1 ). We retrieved a total of 9065 unique citations from database searches, which were reviewed (through screening by title and abstract) along with 416 additional references identified from the thirteen systematic reviews also identified. We selected 145 references for full text revision, from which 35 primary studies (reporting on 24 different interventions) were finally included in our systematic review [ 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 ].
PRISMA flowchart
Characteristics of the included studies
The characteristics of the included studies are summarized in Table 1 and described in detail in Additional file 4 . Most (86%) were published from 2000 onwards. The studies were conducted in six countries: 15 (42%) were conducted in USA [ 37 , 44 , 45 , 46 , 49 , 50 , 51 , 53 , 54 , 55 , 56 , 57 , 58 , 60 , 70 ], 12 (34%) in France [ 38 , 39 , 40 , 41 , 42 , 43 , 63 , 64 , 65 , 66 , 67 , 68 ], 3 (9%) in the Netherlands [ 52 , 62 , 69 ], and 3 (9%) in Canada [ 36 , 59 , 61 ]. The remaining two studies were conducted in Australia [ 47 ], and Italy [ 48 ]. Eleven studies described interventions to support compliance with guidelines on diagnosis and treatment [ 41 , 43 , 52 , 56 , 64 , 65 , 66 , 67 , 68 , 69 , 70 ], 9 focused on treatment only [ 38 , 39 , 40 , 42 , 47 , 48 , 49 , 62 , 63 ], 5 on diagnosis only [ 45 , 51 , 58 , 59 , 60 ], and 7 on screening [ 36 , 37 , 46 , 50 , 54 , 57 , 61 ]. Six studies were randomized controlled trials [ 37 , 45 , 50 , 51 , 54 , 60 ], four were non-randomized controlled trials [ 46 , 57 , 58 , 63 ], eight non-controlled before-after studies [ 42 , 49 , 53 , 55 , 59 , 62 , 65 , 69 ], one prospective cohort study , three cross-sectional studies [ 44 , 47 , 56 ], one mixed-methods [ 36 ] and twelve case studies [ 38 , 39 , 40 , 41 , 43 , 48 , 52 , 61 , 64 , 66 , 67 , 68 ].
Thirty of the 35 studies (85%) evaluated the impact of the interventions on compliance rate [ 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 53 , 54 , 55 , 56 , 58 , 59 , 60 , 62 , 63 , 65 , 66 , 67 , 68 , 69 , 70 ]. Four studies [ 43 , 46 , 50 , 57 ] evaluated the impact on determinants of behavior change related outcomes (providers’ knowledge, attitudes, and self-efficacy about the CPGs recommendations). Two studies evaluated intervention adoption and fidelity [ 36 , 44 ]. No study evaluated the impact of the intervention on patient outcomes, and only one study [ 44 ] evaluated the costs of implementing the interventions.
Characteristics of the interventions to support compliance with breast cancer clinical practice guidelines
Table 2 describes the characteristics of each type of intervention. Twelve studies described two different interventions consisting in the implementation of computerized decision support systems [ 38 , 39 , 40 , 41 , 42 , 43 , 48 , 64 , 65 , 66 , 67 , 68 ], 7 described 6 different educational interventions targeting health care professionals [ 44 , 50 , 55 , 57 , 58 , 59 , 63 ], 9 described 9 multifaceted interventions [ 36 , 37 , 46 , 49 , 51 , 53 , 54 , 60 , 62 ], and two studies described two audit and feedback interventions [ 47 , 69 ]. The rest of the studies described interventions based on: implementation of clinical pathways [ 56 ], integrated knowledge translation systems [ 61 ], medical critiquing system [ 52 ], medical home program [ 70 ], and reminders to providers [ 45 ].
Computerized decision support systems
The use of computerized decision support systems to promote compliance with breast cancer CPGs was described in 12 studies [ 38 , 39 , 40 , 41 , 42 , 43 , 48 , 64 , 65 , 66 , 67 , 68 ]. Eleven of them reported the same intervention, which consisted of a system developed in France called OncoDoc [38, 40–43, 64–68). OncoDoc is a computerized clinical decision support system that provides patient-specific recommendations for breast cancer patients according to CancerEst (local) CPGs [ 71 ]. A study conducted in Italy reported on the development of a similar system, the OncoCure CDSS [ 48 ].
Educational interventions
Seven studies described educational interventions targeting healthcare providers to promote compliance with breast cancer CPGs [ 44 , 50 , 55 , 57 , 58 , 59 , 63 ]. One intervention consisted in the provision of academic detailing on breast cancer screening (based on the American Cancer Society guidelines for the early detection of BC) among primary care physicians in an underserved community in the USA [ 50 ]. An intervention in seven hospitals in France consisted in monthly meetings where local opinion leaders presented the relevant sections of the CPGs, which were subsequently sent to all the participating physicians who were expected to use them in their practice [ 63 ]. Another intervention consisted in a comprehensive continuing medical education package to address pre-identified barriers to guideline adherence. The intervention followed a multimethod approach to physician education including CME conferences, physician newsletters, CBE skills training, BC CME monograph, “question of the month” among hospital staff meetings, primary care office visits, and patient education materials [ 57 , 58 ]. An educational intervention to improve compliance with radiological staging CPGs in early breast cancer patients [ 59 ] consisted of multidisciplinary educational rounds, presenting the Cancer Care Ontario Practice Guidelines [ 72 ]. Another intervention, aimed to support compliance with recommendation against serum tumor marker tests and advanced imaging for BC survivors who are asymptomatic for recurrence, consisted in academic detailing for oncologists at regular meetings [ 55 ]. Another intervention [ 44 ] consisted in an online course to learn to implement and deliver the Strength after Breast Cancer (SABC) guidelines (with recommendations about rehabilitative exercise for breast cancer survivors).
Audit and feedback interventions
We identified two audit and feedback interventions [ 47 , 69 ]. One consisted in sending hospitals a written report with regional benchmark information on nine performance indicators measuring the quality of care based on breast cancer national guidelines [ 69 ]. Healthcare professionals attended sessions twice a year, where an anonymous benchmark was presented for each hospital score compared with the regional mean and the norm scores. Another intervention [ 47 ] audited patients’ medical records according to four agreed indicators. Information from the audit forms was entered into a database, which allowed individualized reports for each participating clinician, providing detailed feedback about their practice, with comparisons across the group and against the agreed criteria.
Other types of single component interventions
Five studies described other strategies to promote compliance with breast cancer CPGs [ 45 , 49 , 52 , 56 , 61 , 70 ]. One intervention consisted on a microcomputer tickler system on the ordering of mammograms [ 45 ]. The system displayed the date of the last mammogram ordered in the “comments” section of the encounter form for each visit. An intervention to support compliance with CPGs follow up recommendations in low-income breast cancer survivors [ 70 ] consisted in the implementation of a medical home program to support primary care case management. Providers and networks participating in this program received a payment per eligible patient per month for care coordination. Another intervention consisted in the implementation of new clinical pathways supplemented by clinical vignettes [ 56 ]. Another intervention consisted in an integrated knowledge translation strategy to be used by guideline developers to improve the uptake of their new CPGs on breast cancer screening [ 61 ]. This integrated knowledge translation strategy was based on the Knowledge to Action Framework [ 73 ], and involved the identification of barriers to knowledge use. An intervention to support compliance with the Dutch breast cancer guideline [ 52 ] consisted of a medical critiquing system (computational method for critiquing clinical actions performed by physicians). The system aimed at providing useful feedback by finding differences between the actual actions and a set of ‘ideal’ actions as described by a CPG.
Multifaceted interventions
We identified nine multifaceted interventions [ 36 , 37 , 46 , 49 , 51 , 53 , 54 , 60 , 62 ]. One intervention to increase compliance with mammography screening [ 37 ] consisted of (i) audit results and a comparison with the network benchmark; (ii) academic detailing of exemplar principles and information from the medical literature; (iii) services of a practice facilitator for 9 months who helped the practitioners design their interventions and facilitate “Plan, Do, Study, Act” processes; and iv) information technology support. In another intervention [ 60 ] to increase screening mammography, primary care providers received (i) a fact sheet providing current information on screening mammography for older women; (ii) telephone follow-up of any questions, and; (iii) copies of a simply written pamphlet on mammography that they could distribute to patients. Another intervention [ 54 ] consisted of biannual feedback to primary care providers regarding compliance with cancer screening CPGs and financial bonuses for “good” performers. Feedback reports documented a site’s scores on each screening measure and a total score across all measures, as well as plan-wide scores for comparison. Another intervention [ 51 ] consisted of an educational intervention accompanied by cue enhancement using mammography chart stickers, and by feedback and token rewards. Another intervention [ 46 ] included (i) use of standardized patients to observe and record healthcare professionals’ performance followed by direct feedback; (ii) newsletters to inform healthcare providers about screening methods; (iii) posters and cards presenting key points about CBE and the importance of routine screening mammograms, and; (iv) patient education materials. An intervention to improve compliance with new CPGs by the American Society for Radiation Oncology (ASTRO) on the proper use of hypofractionation [ 49 ] consisted in implementing five consensus-driven and evidence-based clinical directives to guide adjuvant radiation therapy for breast cancer. Prospective contouring rounds were instituted, wherein the treating physicians presented their directive selection and patient contours for peer-review and consensus opinion. Another intervention combined audit and feedback and education to providers to increase compliance with breast cancer treatment guidelines [ 62 ]. Repeated feedback on the performance of the chemotherapy administration, timing and dosing were given to the participants. The feedback consisted of a demonstration of variation in performance between the different hospitals and the region as a whole. The educational component consisted in four consecutive sessions of discussion about relevant literature that became available in that period regarding chemotherapy dose intensity, sequencing of radiotherapy and the importance of adequate axillary lymph node clearance.
An intervention to promote compliance with new National Comprehensive Cancer Network guidelines from routine testing to omission of ordering complete blood cell count and liver function tests in patients with early breast cancer [ 53 ] involved (i) provision of educational materials; (ii) audit and feedback; (iii) certification; (iv) patient education; (v) financial incentives and (vi) implementation of alerts in the electronic medical records. Another intervention to promote breast cancer screening CPGs [ 36 ] included (i) printed educational materials with the recommendations for breast cancer mammography, (ii) printed educational materials with CPGs recommendations for clinical breast exams and breast self-exams, and (iii) video (12 min) directed at clinicians, exploring strategies for patient discussion around breast cancer screening issues.
Risk of bias
The risk of bias was judged as low in five studies [ 45 , 53 , 54 , 59 , 70 ], moderate in ten [ 36 , 37 , 42 , 44 , 47 , 56 , 57 , 58 , 62 , 63 ], and high in five [ 46 , 49 , 50 , 55 , 65 ]. In four studies [ 51 , 52 , 60 , 69 ] the risk of bias was unclear since there was not enough information available to determine potential biases. We did not assess risk of bias for case studies, due to the lack of appropriate tools available. A detailed description of the risk of bias of the included studies, excluding case studies, is available in Additional file 3 .
Impact of the interventions
Six RCTs [ 37 , 45 , 50 , 51 , 54 , 60 ] and four controlled before-after studies [ 50 , 57 , 58 , 63 ] examined the effectiveness of four provider educational interventions, one intervention based on the use of provider reminders, and five multifaceted interventions. In nine of these interventions (90%), the ultimate goal was to improve compliance with breast cancer screening guidelines. Compliance was uniformly measured in terms of mammography rates (e.g., proportion of eligible women undergoing a mammography screening for breast cancer). Except one multifaceted intervention [ 54 ], the interventions consistently showed relevant beneficial effects (Fig. 2 ).
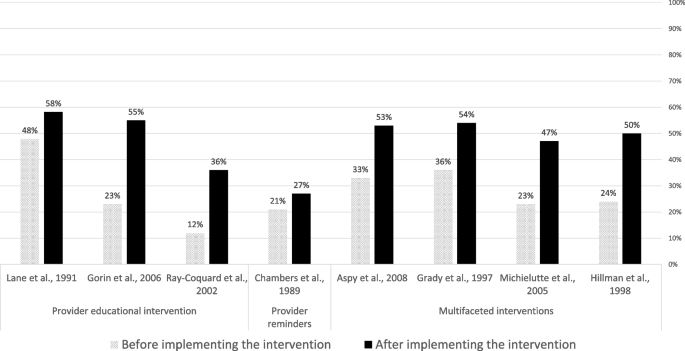
Compliance rate with guideline recommendations before and after the implementation of the identified interventions
Impact of educational interventions
Four studies evaluated the effectiveness of educational interventions targeted to healthcare providers [ 50 , 57 , 58 , 63 ]. A randomized controlled trial showed that the intervention improved recommendation of mammography (odds ratio (OR) 1.85, 95% CI 1.25–2.74) and clinical breast examination (OR 2.13, 95% CI 1.31–3.46) in female patients aged 40 and over [ 50 ]. One controlled before-after study showed significant ( p < 0.05) improvements in providers’ knowledge, attitudes and self-efficacy towards the new CPG screening recommendations [ 57 ], whereas another controlled before-after study reported a significant improvement in the number of reported mammography referrals of asymptomatic women aged 50 to 75 years in the intervention group but not in the control group [ 58 ]. A controlled before-after study observed an improved compliance to diagnostic and treatment CPG recommendations in the intervention group (from 12% before the intervention to 36% post-intervention; P < 0.001), whereas no significant improvements were observed in the control group [ 63 ].
Impact of provider reminders
A randomized controlled trial [ 45 ] showed that a microcomputer-generated reminder system for ordering mammograms improved compliance with mammography guidelines: 27% (170/639) in the intervention vs 21% (128/623) in the control group (OR = 1.40 (95%CI = 1.01 to 1.82); p = 0.011) after 6 months follow-up.
Impact of multifaceted interventions
Five studies examined the impact of multifaceted interventions. A randomized controlled trial observed that, in comparison with usual care, a multifaceted intervention (including audit and feedback; provider education; information technology support) increased the proportion of women offered a mammogram (38% vs 53%), and the proportion of women with a recorded mammogram (35% vs 52%) [ 37 ]. Another trial observed that a multifaceted intervention (comprising provider education and patient education through pamphlets), did not improve compliance with screening mammography guidelines in the overall sample, but produced significant improvements in specific vulnerable subgroups (elderly, lower educational attainment, black ethnicity and with no private insurance) [ 60 ]. A randomized controlled trial observed that a multifaceted intervention (audit and feedback plus financial incentives) doubled screening rates both in the intervention and control groups, with no statistically significant differences observed between groups [ 54 ]. A trial examining a multifaceted intervention (provider education, cue enhancement plus feedback, and token rewards) observed that mammography compliance rates significantly improved ( p < 0.05) in the intervention (62.8%) in comparison with the control (49.0%) group [ 51 ]. A controlled before-after study observed that a multifaceted intervention (including audit and feedback, patient and professional education) improved the demonstration of breast cancer screening, with significantly more women older than 50 receiving mammograms in the intervention than in the comparison group [ 46 ].
Certainty of evidence
The results from the assessment of the certainty of evidence concerning the impact of the interventions on compliance with breast cancer CPGs is available in Additional file 5 . Based on GRADE criteria, we rated the certainty of evidence as “low” for the four educational interventions targeting healthcare providers. This was due to very serious risk of bias, for which we downgraded the level of evidence two levels. For the only intervention identified consisting in a reminder system for healthcare providers, we rated the certainty of evidence as “moderate” (downgrading one level due to serious indirectness). For the five multifaceted interventions, we rated the evidence as “low”, due to serious risk of bias, and serious inconsistency.
Main findings
In this systematic review, we identified 35 studies describing and evaluating the impact of interventions to support clinician compliance with breast cancer CPGs. We described a range of different types of interventions to support adherence of healthcare professionals to breast cancer CPGs. We observed that there is low quality evidence that educational interventions targeted at healthcare professionals may improve compliance with recommendations concerning breast cancer screening, diagnosis and treatment. There is moderate quality of evidence that reminder systems for healthcare professionals improve compliance with recommendations concerning breast cancer screening. There is low quality of evidence that multifaceted interventions may improve compliance with recommendations concerning breast cancer screening. The effectiveness of the remaining types of interventions identified is uncertain, given the study designs available (e.g., cross-sectional, uncontrolled before-after or case studies). There is very limited data on the costs of implementing these interventions.

Strengths and limitations
The main strength of this systematic review is that it addressed a highly relevant question, and provided much needed evidence to help improve providers’ compliance with breast cancer guidelines globally. An additional strength is that, contrary to previous systematic reviews, ours was not limited to experimental studies. By including observational, and qualitative and mixed-methods studies, we were able to provide a richer characterization of the available interventions.
This review has several limitations. First, we restricted the bibliographic searches to peer-reviewed publications in English language only. This may have resulted in failing to identify additional relevant data that could have further informed our assessments of the available evidence. However, we think that the impact of this limitation is likely to be small, as suggested by a recent meta-epidemiologic study [ 74 ]. Second, the heterogeneity of the reporting of outcome data made meta-analysis not feasible. Third, the heterogeneity in outcomes and the large number of strategies used across studies precluded us to determine the unique influence of each strategy on a given outcome.
Our results in the context of previous research
An important finding of our review is that most of the included studies showed that the interventions were effective in improving compliance to CPGs. This is in line with findings from previous, non-condition-specific reviews, which concluded that guideline dissemination and implementation strategies are likely to be efficient [ 75 , 76 ].
A large proportion of the studies included in our review examined the impact of Computerized Decision Support Systems (CDSS). Previous systematic reviews observed that CDSS significantly improve clinical practice [ 77 , 78 ]. In our review, the evidence about CDSS was only available from observational, uncontrolled studies, and was restricted to two tools in France and Italy in the hospital setting. New studies evaluating other CDSS, and in other settings and countries, are therefore needed.
There is substantial evidence from non-condition specific research that audit and feedback interventions can effectively improve quality of care [ 79 ]. A recent systematic review [ 80 ] examining the effectiveness of cancer (all types) guideline implementation strategies showed that providing feedback on CPG compliance was associated with positive significant changes in patient outcomes. More research is needed about the impact of audit and feedback interventions on the compliance with breast cancer CPGs.
Educational interventions targeted to providers (both in isolation and in combination with other interventions) have shown to improve outcomes in patients with cancer [ 80 ]. Despite the low certainty obtained, the studies in our review consistently showed that educational and multifaceted interventions improve compliance with breast cancer CPGs, supporting also results from previous non-condition specific reviews [ 16 , 81 ], as well as current recommendations from the Institute of Medicine [ 82 ].
In line with our finding concerning electronic reminder interventions, a Cochrane systematic review concluded that computer‐generated reminders to healthcare professionals probably improves compliance with preventive guidelines [ 83 ].
Implications for practice and research
In terms of implications for practice, given that compliance with breast cancer guidelines is associated with better survival outcomes [ 25 ], and that there are still a substantial proportion of breast cancer patients not receiving clinical guidelines recommended care [ 21 ], it is important that the most effective interventions available are implemented to improve breast cancer guideline uptake by healthcare providers.
In terms of implications for research, as in a previous non-condition-specific review [ 76 ], we observed that there is very limited data on the costs of implementing the interventions to support compliance with breast cancer CPGs, as well as a scarcity of studies evaluating the effectiveness of interventions targeting the organization of care (e.g., benchmarking tools). Research in these two areas is urgently needed to allow evidence-based decisions concerning which interventions should be rolled out and implemented widely as part of existing quality improvement programs. Also worth noting is that, up to now, the great majority of the research on this (breast cancer) area has focused on measuring the impact of the interventions on process measures (mostly compliance rates). No study measured the impact on patient outcomes, and only a small minority examined the impact on determinants of compliance behavior (e.g., providers’ knowledge, attitudes, or self-efficacy). Future research would benefit from including a broader range of outcomes (including proximal and distal), as this would help to better measure and understand the extent to which the interventions produce the intended benefits.
Future research is also needed to identify the most effective types of interventions in improving CPGs uptake, as well as the “active ingredients” of multifaceted interventions [ 84 ]. The characteristics of the CPGs intended users, and the context in which the clinical practice occurs are likely to be as important as guideline attributes for promoting adoption of CPG recommendations. Therefore, future research should focus on gaining a deeper understanding about how, when, for whom, and under which circumstances the interventions identified can effectively support guideline adherence. Using a realist evaluation methodology [ 85 ] may prove a valuable strategy in this endeavor. However, as observed in our review, the detailed characteristics of the interventions are very frequently scarcely reported. To allow progress in this area, it is of utmost importance that intervention developers and researchers offer in their published reports a comprehensive characterization of their interventions. The Template for Intervention Description and Replication (TIDieR) guidelines [ 86 ] were specifically designed for this purpose.
Promoting the uptake and use of CPGs at the point of care, represents a final translation step, from scientific findings into practice. In this review we identified a wide range of interventions to support adherence of healthcare professionals to breast cancer CPGs. Most of them are based on computerized decision support systems, provision of education, and audit and feedback, which are delivered either in isolation or in combination with other co-interventions. The certainty of evidence is low for educational interventions. The evidence is moderate for automatic reminder systems, and low for multifaceted interventions. For the rest of the interventions identified, the evidence is uncertain. Future research is very much needed to strengthen the available evidence base, concerning not only their impact on compliance, but also on patient important outcomes, and on their cost-effectiveness.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
American Society for Radiation Oncology
Computerized Decision Support Systems
Clinical Practice Guidelines
Effective Practice and Organization Care
Hazard ratio
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Randomized controlled trial
Strength after Breast Cancer
Template for Intervention Description and Replication
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49.
Article PubMed Google Scholar
Bozzi LM, Stuart B, Onukwugha E, Tom SE. Utilization of screening mammograms in the medicare population before and after the affordable care act implementation. J Aging Health. 2020;32(1):25–32.
Sestak I, Cuzick J. Update on breast cancer risk prediction and prevention. Curr Opin Obstet Gynecol. 2015;27(1):92–7.
van Luijt PA, Fracheboud J, Heijnsdijk EA, den Heeten GJ, de Koning HJ. Nation-wide data on screening performance during the transition to digital mammography: observations in 6 million screens. Eur J Cancer (Oxford, England : 1990). 2013;49(16):3517–25.
Article Google Scholar
Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. NEngl J Med. 2005;353(17):1784–92.
Article CAS Google Scholar
Gangnon RE, Stout NK, Alagoz O, Hampton JM, Sprague BL, Trentham-Dietz A. Contribution of breast cancer to overall mortality for US women. Med Decis Mak. 2018;38(1_suppl):24s–31s.
Kalager M, Zelen M, Langmark F, Adami HO. Effect of screening mammography on breast-cancer mortality in Norway. N Engl J Med. 2010;363(13):1203–10.
Article CAS PubMed Google Scholar
Grol R, Grimshaw J. Evidence-based implementation of evidence-based medicine. Jt Comm J Qual Improve. 1999;25(10):503–13.
CAS Google Scholar
Green LA, Seifert CM. Translation of research into practice: why we can’t “just do it.” J Am Board Fam Pract. 2005;18(6):541–5.
Westfall JM, Mold J, Fagnan L. Practice-Based Research—“Blue Highways” on the NIH Roadmap. JAMA. 2007;297(4):403–6.
Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–65.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clin Res ed). 2008;336(7650):924–6.
Bero LA, Grilli R, Grimshaw JM, Harvey E, Oxman AD, Thomson MA. Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. The Cochrane Effective Practice and Organization of Care Review Group. BMJ. 1998;317(7156):465–8.
Article CAS PubMed PubMed Central Google Scholar
Flodgren G, O’Brien MA, Parmelli E, Grimshaw JM. Local opinion leaders: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2019;6(6):Cd000125.
PubMed Google Scholar
Grimshaw J, Eccles M, Thomas R, MacLennan G, Ramsay C, Fraser C, et al. Toward evidence-based quality improvement. Evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966-1998. J Gen Internal Med. 2006;21 Suppl 2(Suppl 2):S14-20.
Google Scholar
Grimshaw JM, Shirran L, Thomas R, Mowatt G, Fraser C, Bero L, et al. Changing provider behavior: an overview of systematic reviews of interventions. Med Care. 2001;39(8 Suppl 2):Ii2-45.
CAS PubMed Google Scholar
Henry NL, Hayes DF, Ramsey SD, Hortobagyi GN, Barlow WE, Gralow JR. Promoting quality and evidence-based care in early-stage breast cancer follow-up. J Natl Cancer Institute. 2014;106(4):dju034.
Keikes L, van Oijen MGH, Lemmens V, Koopman M, Punt CJA. Evaluation of guideline adherence in colorectal cancer treatment in The Netherlands: a survey among medical oncologists by the Dutch Colorectal Cancer Group. Clin Colorectal Cancer. 2018;17(1):58–64.
Subramanian S, Klosterman M, Amonkar MM, Hunt TL. Adherence with colorectal cancer screening guidelines: a review. Prev Med. 2004;38(5):536–50.
Carpentier MY, Vernon SW, Bartholomew LK, Murphy CC, Bluethmann SM. Receipt of recommended surveillance among colorectal cancer survivors: a systematic review. J Cancer Survivorship. 2013;7(3):464–83.
Niño de Guzmán E, Song Y, Alonso-Coello P, Canelo-Aybar C, Neamtiu L, Parmelli E, et al. Healthcare providers’ adherence to breast cancer guidelines in Europe: a systematic literature review. Breast Cancer Res Treat. 2020;181(3):499–518.
Article PubMed PubMed Central Google Scholar
Ament SM, de Groot JJ, Maessen JM, Dirksen CD, van der Weijden T, Kleijnen J. Sustainability of professionals’ adherence to clinical practice guidelines in medical care: a systematic review. BMJ Open. 2015;5(12): e008073.
Ferron G, Martinez A, Gladieff L, Mery E, David I, Delannes M, et al. Adherence to guidelines in gynecologic cancer surgery. Int J Gynecol Cancer. 2014;24(9):1675–8.
Bahtsevani C, Uden G, Willman A. Outcomes of evidence-based clinical practice guidelines: a systematic review. Int J Technol Assess Health Care. 2004;20(4):427–33.
Ricci-Cabello I, Vásquez-Mejía A, Canelo-Aybar C, Niño de Guzman E, Pérez-Bracchiglione J, Rabassa M, et al. Adherence to breast cancer guidelines is associated with better survival outcomes: a systematic review and meta-analysis of observational studies in EU countries. BMC Health Serv Res. 2020;20(1):920.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355: i4919.
The National Institutes of Health (NIH). Quality assessment tool for before-after (Pre-Post) study with no control group. 2021. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools . Last Accessed 4 Feb 2022 .
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open. 2016;6(12): e011458.
Pace R, Pluye P, Bartlett G, Macaulay AC, Salsberg J, Jagosh J, et al. Testing the reliability and efficiency of the pilot Mixed Methods Appraisal Tool (MMAT) for systematic mixed studies review. Int J Nurs Stud. 2012;49(1):47–53.
Effective Practice and Organisation of Care (EPOC). EPOC Taxonomy; 2015. epoc.cochrane.org/epoc-taxonomy (Accessed 11 May 2022).
EPOC. Cochrane Effective Practice and Organisation of Care (EPOC). EPOC Resources for review authors, 2017. epoc.cochrane.org/resources/epoc-resources-review-authors (Accessed 9 Feb 2023). 2017.
Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2.
Armson H, Roder S, Elmslie T, Khan S, Straus SE. How do clinicians use implementation tools to apply breast cancer screening guidelines to practice? Implement Sci. 2018;13(1):79.
Aspy CB, Enright M, Halstead L, Mold JW. Improving mammography screening using best practices and practice enhancement assistants: an Oklahoma Physicians Resource/Research Network (OKPRN) study. J Am Board Fam Med. 2008;21(4):326–33.
Bouaud J, Blaszka-Jaulerry B, Zelek L, Spano JP, Lefranc JP, Cojean-Zelek I, et al. Health information technology: use it well, or don’t! Findings from the use of a decision support system for breast cancer management. AMIA Annu SympProc AMIA Symp. 2014;2014:315–24.
Bouaud J, Pelayo S, Lamy JB, Prebet C, Ngo C, Teixeira L, et al. Implementation of an ontological reasoning to support the guideline-based management of primary breast cancer patients in the DESIREE project. Artificial Intell Med. 2020;108: 101922.
Bouaud J, Seroussi B. Impact of site-specific customizations on physician compliance with guidelines. Stud Health Technol Inform. 2002;90:543–7.
Bouaud J, Seroussi B. Revisiting the EBM decision model to formalize non-compliance with computerized CPGs: results in the management of breast cancer with OncoDoc2. AMIA Annu Symp Proc AMIA Symp. 2011;2011:125–34.
Bouaud J, Seroussi B, Antoine EC, Zelek L, Spielmann M. A before-after study using OncoDoc, a guideline-based decision support-system on breast cancer management: impact upon physician prescribing behaviour. Stud Health Technol Inform. 2001;84(Pt 1):420–4.
Bouaud J, Spano JP, Lefranc JP, Cojean-Zelek I, Blaszka-Jaulerry B, Zelek L, et al. Physicians’ attitudes towards the advice of a guideline-based decision support system: a case study with OncoDoc2 in the management of breast cancer patients. Stud Health Technol Inform. 2015;216:264–9.
Calo WA, Doerksen SE, Spanos K, Pergolotti M, Schmitz KH. Implementing Strength after Breast Cancer (SABC) in outpatient rehabilitation clinics: mapping clinician survey data onto key implementation outcomes. Implement Sci Commun. 2020;1:69.
Chambers CV, Balaban DJ, Carlson BL, Ungemack JA, Grasberger DM. Microcomputer-generated reminders. Improving the compliance of primary care physicians with mammography screening guidelines. J Fam Pract. 1989;29(3):273–80.
Coleman EA, Lord J, Heard J, Coon S, Cantrell M, Mohrmann C, et al. The Delta project: increasing breast cancer screening among rural minority and older women by targeting rural healthcare providers. Oncol Nurs Forum. 2003;30(4):669–77.
Craft PS, Zhang Y, Brogan J, Tait N, Buckingham JM. Implementing clinical practice guidelines: a community-based audit of breast cancer treatment. Med J Aust. 2000;172(5):213–6.
Eccher C, Seyfang A, Ferro A. Implementation and evaluation of an Asbru-based decision support system for adjuvant treatment in breast cancer. Comput Methods Programs Biomed. 2014;117(2):308–21.
Gilbo P, Potters L, Lee L. Implementation and utilization of hypofractionation for breast cancer. Adv Radiation Oncol. 2018;3(3):265–70.
Gorin SS, Ashford AR, Lantigua R, Hossain A, Desai M, Troxel A, et al. Effectiveness of academic detailing on breast cancer screening among primary care physicians in an underserved community. J Am Board Fam Med. 2006;19(2):110–21.
Grady KE, Lemkau JP, Lee NR, Caddell C. Enhancing mammography referral in primary care. Prev Med. 1997;26(6):791–800.
Groot P, Hommersom A, Lucas PJ, Merk RJ, ten Teije A, van Harmelen F, et al. Using model checking for critiquing based on clinical guidelines. Artificial Intell Med. 2009;46(1):19–36.
Hill LA, Vang CA, Kennedy CR, Linebarger JH, Dietrich LL, Parsons BM, et al. A strategy for changing adherence to national guidelines for decreasing laboratory testing for early breast cancer patients. Wisconsin Med J. 2018;117(2):68–72.
Hillman AL, Ripley K, Goldfarb N, Nuamah I, Weiner J, Lusk E. Physician financial incentives and feedback: failure to increase cancer screening in Medicaid managed care. Am J Public Health. 1998;88(11):1699–701.
Kreizenbeck KL, Wong T, Jagels B, Smith JC, Irwin BB, Jensen B, et al. A pilot study to increase adherence to ASCO Choosing Wisely recommendations for breast cancer surveillance at community clinics. J Clin Oncol. 2020;38(29_suppl):18.
Kubal T, Peabody JW, Friedman E, Levine R, Pursell S, Letson DG. Using vignettes to measure and encourage adherence to clinical pathways in a quality-based oncology network: an early report on the moffitt oncology network initiative. Managed Care (Langhorne, Pa). 2015;24(10):56–64.
Lane DS, Messina CR, Grimson R. An educational approach to improving physician breast cancer screening practices and counseling skills. Patient Educ Counsel. 2001;43(3):287–99.
Lane DS, Polednak AP, Burg MA. Effect of continuing medical education and cost reduction on physician compliance with mammography screening guidelines. J Family Pract. 1991;33(4):359–68.
McWhirter E, Yogendran G, Wright F, Pharm GD, Clemons M. Baseline radiological staging in primary breast cancer: impact of educational interventions on adherence to published guidelines. J Eval Clin Pract. 2007;13(4):647–50.
Michielutte R, Sharp PC, Foley KL, Cunningham LE, Spangler JG, Paskett ED, et al. Intervention to increase screening mammography among women 65 and older. Health Educ Res. 2005;20(2):149–62.
Munce S, Kastner M, Cramm H, Lal S, Deschene SM, Auais M, et al. Applying the knowledge to action framework to plan a strategy for implementing breast cancer screening guidelines: an interprofessional perspective. J Cancer Educ. 2013;28(3):481–7.
Ottevanger PB, De Mulder PH, Grol RP, van Lier H, Beex LV. Adherence to the guidelines of the CCCE in the treatment of node-positive breast cancer patients. Eur J Cancer (Oxford, England : 1990). 2004;40(2):198–204.
Ray-Coquard I, Philip T, de Laroche G, Froger X, Suchaud JP, Voloch A, et al. A controlled “before-after” study: impact of a clinical guidelines programme and regional cancer network organization on medical practice. Br J Cancer. 2002;86(3):313–21.
Seroussi B, Bouaud J, Antoine EC. ONCODOC: a successful experiment of computer-supported guideline development and implementation in the treatment of breast cancer. Artificial Intell Med. 2001;22(1):43–64.
Seroussi B, Bouaud J, Gligorov J, Uzan S. Supporting multidisciplinary staff meetings for guideline-based breast cancer management: a study with OncoDoc2. AMIA Annu Symp Proc AMIA Symp. 2007:656-60.
Seroussi B, Laouenan C, Gligorov J, Uzan S, Mentre F, Bouaud J. Which breast cancer decisions remain non-compliant with guidelines despite the use of computerised decision support? Br J Cancer. 2013;109(5):1147–56.
Seroussi B, Soulet A, Messai N, Laouenan C, Mentre F, Bouaud J. Patient clinical profiles associated with physician non-compliance despite the use of a guideline-based decision support system: a case study with OncoDoc2 using data mining techniques. AMIA Annu Symp Proc AMIA Symp. 2012;2012:828–37.
Seroussi B, Soulet A, Spano JP, Lefranc JP, Cojean-Zelek I, Blaszka-Jaulerry B, et al. Which patients may benefit from the use of a decision support system to improve compliance of physician decisions with clinical practice guidelines: a case study with breast cancer involving data mining. Stud Health Technol Inform. 2013;192:534–8.
Veerbeek L, van der Geest L, Wouters M, Guicherit O, Does-den Heijer A, Nortier J, et al. Enhancing the quality of care for patients with breast cancer: seven years of experience with a Dutch auditing system. Eur J Surg Oncol. 2011;37(8):714–8.
Wheeler SB, Kohler RE, Goyal RK, Lich KH, Lin CC, Moore A, et al. Is medical home enrollment associated with receipt of guideline-concordant follow-up care among low-income breast cancer survivors? Med Care. 2013;51(6):494–502.
CancerEst. Principes de prise en charge des cancers du sein en situation non me´tastatique: le re´fe´rentiel CancerEst. 2008.
Nofech-Mozes S, Vella ET, Dhesy-Thind S, Hanna WM. Cancer care Ontario guideline recommendations for hormone receptor testing in breast cancer. Clin Oncol (Royal College of Radiologists (Great Britain)). 2012;24(10):684–96.
Graham ID, Logan J, Harrison MB, Straus SE, Tetroe J, Caswell W, et al. Lost in knowledge translation: time for a map? J Continuing Educ Health Prof. 2006;26(1):13–24.
Nussbaumer-Streit B, Klerings I, Dobrescu AI, Persad E, Stevens A, Garritty C, et al. Excluding non-English publications from evidence-syntheses did not change conclusions: a meta-epidemiological study. J Clin Epidemiol. 2020;118:42–54.
Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess (Winchester, England). 2004;8(6):iii–iv, 1–72.
Flodgren G, Hall AM, Goulding L, Eccles MP, Grimshaw JM, Leng GC, et al. Tools developed and disseminated by guideline producers to promote the uptake of their guidelines. Cochrane Database Syst Rev. 2016;8:Cd010669.
Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765.
Garg AX, Adhikari NK, McDonald H, Rosas-Arellano MP, Devereaux PJ, Beyene J, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293(10):1223–38.
Ivers NM, Grimshaw JM, Jamtvedt G, Flottorp S, O’Brien MA, French SD, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Internal Med. 2014;29(11):1534–41.
Tomasone JR, Kauffeldt KD, Chaudhary R, Brouwers MC. Effectiveness of guideline dissemination and implementation strategies on health care professionals’ behaviour and patient outcomes in the cancer care context: a systematic review. Implement Sci. 2020;15(1):41.
Giguère A, Zomahoun HTV, Carmichael PH, Uwizeye CB, Légaré F, Grimshaw JM, et al. Printed educational materials: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2020;8(8):Cd004398.
Greenfield S, Steinberg E, Auerbach A, Avorn J, Galvin R, Gibbons R. Clinical practice guidelines we can trust. Washington, DC: Institute of Medicine; 2011. p. 2017.
Arditi C, Rège-Walther M, Durieux P, Burnand B. Computer-generated reminders delivered on paper to healthcare professionals: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2017;7(7):Cd001175.
Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337: a1655.
Pawson R, Tilley N, Tilley N. Realistic evaluation: sage; 1997.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348: g1687.
Download references
Acknowledgements
Not applicable.
The systematic review was carried out by the Iberoamerican Cochrane Center under Framework contract 443094 for procurement of services between European Commission Joint Research Centre and Asociación Colaboración Cochrane Iberoamericana.
Author information
Authors and affiliations.
Balearic Islands Health Research Institute (IdISBa), Palma, Spain
Ignacio Ricci-Cabello
Primary Care Research Unit of Mallorca, Balearic Islands Health Service, Palma, Spain
CIBER de Epidemiología y Salud Pública (CIBERESP), Madrid, Spain
Ignacio Ricci-Cabello, Pablo Alonso-Coello & Ivan Solà
Facultad de Medicina Humana, Universidad Nacional Mayor de San Marcos, Lima, Peru
Darla Carvallo-Castañeda & Adrián Vásquez-Mejía
Iberoamerican Cochrane Centre-Department of Clinical Epidemiology and Public Health, Biomedical Research Institute Sant Pau (IIB Sant Pau), Barcelona, Spain
Pablo Alonso-Coello, David Rigau, Ivan Solà & Ena Niño-de-Guzmán
European Commission, Joint Research Centre (JRC), Ispra, Italy
Zuleika Saz-Parkinson, Elena Parmelli, Gian Paolo Morgano & Luciana Neamtiu
Cancer Prevention and Control Programme, Catalan Institute of Oncology, IDIBELL, Hospitalet de Llobregat, Barcelona, Spain
Ena Niño-de-Guzmán
You can also search for this author in PubMed Google Scholar
Contributions
EP, ZSP, and LN contributed to conception and design of the study. IS designed the literature search. IRC ENG, LN, ZSP, EP, DC, APVM, and GPM performed the literature screening, data extraction, and quality appraisal of included studies. PAC and IRC evaluated the certainty of evidence. All authors contributed to data interpretation. IRC wrote the first draft of the manuscript. All authors critically reviewed and revised the manuscript and approved the final manuscript.
Corresponding authors
Correspondence to Pablo Alonso-Coello or Elena Parmelli .
Ethics declarations
Ethics approval and consent to participate.
Not applicable (literature review).
Consent for publication
Not applicable (the manuscript does not contain data from any individual person).
Competing interests
EP, LN, and ZSP are or were employees of the Joint Research Centre, European Commission. ENDG, DR, IS, and PAC are employees of the Iberoamerican Cochrane Center.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
PRISMA 2020 Checklist.
Additional file 2.
Search strategy.
Additional file 3.
Summary of Risk of Bias Assessment.
Additional file 4.
Characteristics and results of the 35 studies included in the review.
Additional file 5.
Evidence Profiles.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ricci-Cabello, I., Carvallo-Castañeda, D., Vásquez-Mejía, A. et al. Characteristics and impact of interventions to support healthcare providers’ compliance with guideline recommendations for breast cancer: a systematic literature review. Implementation Sci 18 , 17 (2023). https://doi.org/10.1186/s13012-023-01267-2
Download citation
Received : 17 May 2022
Accepted : 14 March 2023
Published : 22 May 2023
DOI : https://doi.org/10.1186/s13012-023-01267-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Clinical guidelines
- Interventions
- Systematic literature review
Implementation Science
ISSN: 1748-5908
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Vitacrystallography: structural biomarkers of breast cancer obtained by x-ray scattering.

Simple Summary
Share and cite.
Denisov, S.; Blinchevsky, B.; Friedman, J.; Gerbelli, B.; Ajeer, A.; Adams, L.; Greenwood, C.; Rogers, K.; Mourokh, L.; Lazarev, P. Vitacrystallography: Structural Biomarkers of Breast Cancer Obtained by X-ray Scattering. Cancers 2024 , 16 , 2499. https://doi.org/10.3390/cancers16142499
Denisov S, Blinchevsky B, Friedman J, Gerbelli B, Ajeer A, Adams L, Greenwood C, Rogers K, Mourokh L, Lazarev P. Vitacrystallography: Structural Biomarkers of Breast Cancer Obtained by X-ray Scattering. Cancers . 2024; 16(14):2499. https://doi.org/10.3390/cancers16142499
Denisov, Sergey, Benjamin Blinchevsky, Jonathan Friedman, Barbara Gerbelli, Ash Ajeer, Lois Adams, Charlene Greenwood, Keith Rogers, Lev Mourokh, and Pavel Lazarev. 2024. "Vitacrystallography: Structural Biomarkers of Breast Cancer Obtained by X-ray Scattering" Cancers 16, no. 14: 2499. https://doi.org/10.3390/cancers16142499
Article Metrics
Further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMC Womens Health
- PMC11055241

Factors influencing breast cancer screening practices among women worldwide: a systematic review of observational and qualitative studies
Banafsheh tavakoli.
1 Student Research Committee, School of Health, Isfahan University of Medical Sciences, Isfahan, Iran
2 Department of Epidemiology and Biostatistics, Isfahan University of Medical Sciences, Isfahan, Iran
Fereshteh Zamani-Alavijeh
3 Department of Health Education and Health Promotion, School of Health, Isfahan University of Medical Sciences, Isfahan, Iran
Hossein Shahnazi
Associated data.
This published article and its supplementary information files include all data generated or analyzed during this study.
The variation in breast cancer incidence rates across different regions may reflect disparities in breast cancer screening (BCS) practices. Understanding the factors associated with these screening behaviors is crucial for identifying modifiable elements amenable to intervention. This systematic review aims to identify common factors influencing BCS behaviors among women globally.
Relevant papers were sourced from PubMed, Scopus, Embase, and Google Scholar. The included studies were published in English in peer-reviewed journals from January 2000 to March 2023 and investigated factors associated with BCS behaviors.
From an initial pool of 625 articles, 34 studies (comprising 29 observational and 5 qualitative studies) with 36,043 participants were included. Factors influencing BCS behaviors were categorized into nine groups: socio-demographic factors, health status history, knowledge, perceptions, cultural factors, cues to action, motivation, self-efficacy, and social support. The quality appraisal scores of the studies ranged from average to high.
Conclusions
This systematic review highlights factors pivotal for policy-making at various levels of breast cancer prevention and assists health promotion professionals in designing more effective interventions to enhance BCS practices among women.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12905-024-03096-x.
Breast cancer stands as the most commonly diagnosed cancer among women worldwide, affecting both developed and developing countries [ 1 ]. Statistical analyses indicate that while wealthier nations report higher breast cancer incidence rates, less developed countries suffer from higher relative mortality rates [ 2 ].
In high-income countries, including the United Kingdom, Australia, and Eastern Europe, over 60% of women are diagnosed at stages one and two of the disease, significantly improving their survival rates. Conversely, women in low-income countries often seek treatment at advanced disease stages when it has metastasized to other organs [ 3 ].
Differences in cancer incidence rates across populations may be attributable to the variance in risk factor prevalence and the implementation or uptake of screening programs [ 4 ].
Routine screening is pivotal in detecting breast cancer at an early, more treatable stage, significantly reducing mortality rates [ 5 ]. The primary methods of screening include breast self-examination (BSE), clinical breast examination (CBE) by a healthcare professional, and mammography (MMG), all of which have been demonstrated to lower mortality rates from breast cancer in various studies [ 6 – 9 ].
Despite numerous interventions and educational efforts aimed at promoting participation in BCS programs, recent studies indicate a continuing rise in mortality rates and a persistently low participation rate among women, particularly in less developed countries [ 1 , 10 ]. For instance, recent figures show that only 13.6% of Malaysian, 0.3% of Egyptian, and 3.8% of Ethiopian women have undergone MMG in the past two years, compared to 81%, 88%, and 70% in Belgium, Australia, and the United States, respectively [ 11 – 16 ]. These disparities highlight the crucial need for developing and implementing effective strategies based on scientific and reliable research to enhance screening behaviors across different societies.
Given the significance of BCS and the dire predictions that both morbidity and mortality from breast cancer will more than double by 2035 [ 3 ], it becomes imperative to conduct a comprehensive review of the published literature. This systematic review aims to [ 1 ] summarize current knowledge on factors influencing BCS behaviors and [ 2 ] identify factors relevant to enhancing screening behaviors among women worldwide. Achieving these objectives and leveraging the findings of this research could empower policymakers, researchers, and health promotion professionals to devise more effective prevention policies and interventions, thereby improving BCS behaviors through well-informed strategies.
This systematic review was registered with PROSPERO under the registration number CRD42023432810. The presentation of findings adheres to the PRISMA checklist standards (Additional file 1 ).
Search Strategy
The research question, structured according to the PICOS framework, was: “What are the factors impacting BCS behaviors among women worldwide?”
The PICOS elements defined were as follows:
- Population: Healthy individuals aged 15 years or older, encompassing all genders, races, and geographic locations.
- Intervention (Influential Factors): This includes socio-demographic factors, health history, knowledge, perceptions, cultural factors, cues to action, motivation, self-care, and social support.
- Comparison Group: Subpopulations and subgroups differentiated by socio-demographic variables.
- Outcome: Practices related to BCS.
- Study Design: The review included cross-sectional, retrospective, prospective, and qualitative studies.
Four key search concepts and their synonyms (Table 1 ) were identified for the search. The international databases searched included PubMed, Scopus, Science Direct, Embase, and Google Scholar. Berenguer and Sakellariou’s search strategy [ 17 ] was adopted. The search concepts, along with their synonyms (utilizing truncations and wildcards, as indicated in Tables 1 and Additional file 2 ), where the asterisk ‘*’ was applied where appropriate, and subject heading terms were combined using the Boolean operators ‘OR’ within concepts, and ‘AND’ to combine concepts, thus developing the final search strategy (Additional file 2 ).
Search key terms achieved from the research question
| Factor | Associat* | Participate* | Breast cancer screening practices |
|---|---|---|---|
| Determin* | Relat* | Adherence* | Breast cancer screening behaviors |
| Predict* | Impact | Attendance* | Breast cancer screening programs |
| Barrier | Dependent | Uptake | Breast cancer prevent* programs |
| Enabler | Affect | Breast cancer screen* | |
| Facilitator | Mammogra* | ||
| Clinical breast exam* | |||
| Breast self-exam* |
*Some letters have been added to these words in the search time
Inclusion and exclusion criteria
Studies were included if they:
- Reported on MMG, CBE, or BSE as methods for BCS, in alignment with recommendations by international health organizations.
- Were published in peer-reviewed journals between January 2000 and March 2023.
- Addressed factors associated with BCS behaviors, focusing on associated factors rather than the effects of interventions.
- Employed quantitative or qualitative research designs.
- Included participants aged 15 years or older.
The exclusion criteria for the studies were:
- Duplicate publications across databases.
- Non-original research articles, including dissertations, reviews, case reports, editorials, oral and poster presentations, and book chapters.
- Publications in languages other than English.
- Preprints are not subjected to peer review.
- Studies focusing on general cancer screening are not specific to breast cancer.
- The research concentrated on other preventative behaviors or early detection methods unrelated to BCS.
- Studies focused on factors associated with the second BCS participation round.
- Research involving women with specific conditions, such as those who are sick or vulnerable.
Study selection
The selection followed PRISMA guidelines. Initially, duplicates across databases were removed. Titles and abstracts were then reviewed for relevance, and articles not meeting the inclusion criteria were discarded. Subsequently, full texts of the remaining studies were evaluated for relevance, with any further non-compliant studies excluded. This review process was independently conducted by two researchers, with any discrepancies resolved through discussion.
Quality assessment
Following numerous academics’ recommendations, the methodological quality of the included studies was assessed, and a Methodological Quality Score (MQS) was assigned. Experts evaluated each study’s conceptual and methodological rigor, resolving discrepancies by consensus. Based on Bernstein’s standards [ 18 ] and as explained by Patton [ 19 ], the assessment criteria included theoretical framework usage, study design, sample size, measurement instruments, data analysis, and reporting on reliability and validity. Quantitative studies were scored on a scale from 0 to 19, and qualitative studies from 0 to 14, with higher scores indicating higher methodological quality. Studies scoring below 60% were excluded.
Data extraction and synthesis
Data were independently extracted by two researchers (BT and HSH), using a pre-designed tool to collect methodological details, including first author, publication year, study design, data source, study location, sampling strategy, sample size, data collection techniques, participant age, BCS method, and conceptual framework. For quantitative studies, additional data on screening participation rates and identified factors associated with BCS behaviors were noted. Qualitative studies included thematic information extracted for analysis.
An initial search yielded 625 articles from the specified databases. After removing duplicates and screening titles and abstracts, 118 papers were selected for full-text evaluation. Ultimately, 34 papers comprising 29 observational studies and 5 qualitative studies, with 36,043 participants, were included in the final review. The study selection process is illustrated in Fig. 1 .

PRISMA flow diagram of the study selection procedure
Quality of included studies
None of the studies achieved the highest possible score. A majority of the studies were cross-sectional designs (82.4%), and over half (64.7%) included large samples (more than 300 participants). Furthermore, 67.7% of the studies grounded their findings in specific theoretical frameworks. Approximately half reported the psychometric properties of their assessment instruments. A significant portion (85.3%, N = 29) of the studies were quantitative and utilized both descriptive and advanced statistical analyses, such as t-tests, multiple regression, logistic regression, and multivariate analysis. The qualitative studies (14.7%, N = 5) primarily employed content and thematic analysis. All quantitative studies assessed the statistical significance of factors associated with BCS behaviors (Table 2 ).
Criteria for methodological quality assessment of reviewed studies and the frequency distributions of each criterion
| Methodological Characteristic | Scoring Options | Frequency (n) | Percent (%) |
|---|---|---|---|
| Theoretical Framework | The study had no theory = 0 points | 11 | 32.3 |
| The study was based on a specific theory = 2 points | 23 | 67.7 | |
| Design | |||
| Study Design | Cross-sectional = 1 point | 28 | 82.4 |
| Retrospective = 2 points | 0 | 0 | |
| Prospective = 3 points | 1 | 2.9 | |
| Qualitative = 3 point | 5 | 14.7 | |
| Sample and measures | |||
| Sample size | Small sample (< 100) = 1 point | 8 | 23.5 |
| Medium sample (> 100 and < 300) = 2 points | 4 | 11.8 | |
| Large sample (> 300) = 3 points | 22 | 64.7 | |
| Measuring Instrument | Not reported = 0 point | 0 | 0 |
| Authors developed the instrument measuring factors = 1 point | 15 | 44.1 | |
| Authors adopted a previously established instrument = 2 points | 19 | 55.9 | |
| Analytical approaches | |||
| Data analysis | Univariate statistics/descriptive = 1 point | 2 | 5.9 |
| Bivariate statistics/ANOVA = 2 points | 1 | 2.9 | |
| Multiple/logistic regression/ANCOVA = 3 points | 24 | 70.6 | |
| Qualitative analysis (content & thematic analysis) = 3points | 5 | 14.7 | |
| Multivariate statistics (structural equation modeling) = 4 points | 2 | 5.9 | |
| Reliability | Not reported = 0 points | 19 | 55.9 |
| Reported = 1 point | 15 | 44.1 | |
| Validity | Not reported = 0 points | 19 | 55.9 |
| Reported = 1 point | 15 | 44.1 | |
| Results | |||
| Factors Associated with BC Screening | No factors were identified = 0 points | ||
| Uncontrolled analysis (factors were not tested for statistical significance) = 1 point | 5 | 14.7 | |
| Controlled analysis (factors were tested for statistical significance) = 2 points | 29 | 85.3 | |
| Conclusions | Not appropriate = 0 points | ||
| Appropriate = 1 point | 34 | 100 |
Characteristics of included studies
The 34 articles that met the inclusion and exclusion criteria were geographically diverse: 20 studies were conducted in Asia [ 10 , 11 , 20 – 37 ], 5 in America [ 16 , 38 – 41 ], 4 in Europe [ 14 , 42 – 44 ], 4 in Africa [ 12 , 13 , 45 , 46 ], and 1 in Australia [ 15 ].
The sample sizes ranged from 8 to 11,409 participants, with the age of participants spanning from 15 to 82 years. Except for one qualitative study focusing on Arab men’s perceptions of female BCS [ 34 ], all participants were women.
There was variability in the BCS methods and the measurement of related factors across studies. Eleven studies identified CBE, BSE, or MMG as the screening methods [ 13 , 20 , 22 , 29 , 30 , 32 , 34 , 36 , 37 , 41 , 46 ]; four defined BSE or MMG [ 12 , 25 , 31 , 35 ]; one mentioned CBE or MMG [ 11 ]; one mentioned CBE or BSE [ 24 ]; one specified CBE alone [ 39 ]; six identified BSE alone [ 23 , 26 , 28 , 33 , 45 , 47 ]; and ten focused solely on MMG [ 14 – 16 , 27 , 38 , 40 , 42 – 44 , 48 ].
The reported BCS rates varied significantly across studies, from 0.3 to 62% for BSE, 2.5–41% for CBE, and 0.3–88.1% for MMG (Table 3 ).
Summary of the characteristics of included studies reviewed
| Author, Year | Study design | Data source | Country | Sampling method | Sample size | Participants and age of participants | Screening method and Screening participation rate | Conceptual framework | MQS |
|---|---|---|---|---|---|---|---|---|---|
| Safarpour et al., 2018 [ ] | Cross-sectional | Questionnaire | Iran | Simple Random | 304 | Women 20–65 | BCS (BSE or CBE):17.1% | Knowledge-Attitude Practice Model | 15 |
| Moreira et al., 2018 [ ] | Cross-sectional | Questionnaire | Brazil | Convenient | 40 | Women 50–69 | MMG: Not Reported | Health Belief Model | 12 |
| Dewi et al., 2019 [ ] | Cross-sectional | Questionnaire | Indonesia | Multistage, Stratified And Cluster, Random | 1967 | Women 20–60 | BSE: 44.4% | Health Belief Model | 15 |
| Fouladi et al., 2013 [ ] | Cross sectional | Questionnaire | Iran | Convenient | 380 | Women ≥ 30 | BSE: 27% MMG: 6.8% | Health Belief Model | 14 |
| Canbulat and Uzun, 2008 [ ] | Cross-sectional | Questionnaire | Turkey | Stratified And Systematic | 268 | Women ≥ 20 | BSE: 21.9% MMG: 12.5% | Health Belief Model | 14 |
| Ahmad and Stewart, 2004 [ ] | Cross-sectional | Questionnaire | Canada | Convenient | 54 | Women 25–60 | CBE: 38.5% | Health Belief Model | 11 |
| Harirchi et al., 2012 [ ] | Cross-sectional | Questionnaire | Iran | Stratified Simple-Random | 770 | Women ≥ 30 | BSE: 36.6% CBE:17.4% MMG: 6.4% | Knowledge-Attitude Practice Model | 13 |
| Kardan-Souraki et al., 2019 [ ] | Cross sectional | Questionnaire | Iran | Cluster | 1165 | Women ≥ 30 | BSE: 62% CBE:41.1% MMG: 21.7% | None | 13 |
| Bailly et al., 2023 [ ] | Cross-sectional | Statistical information units | France | Stratified Random | 144 | Women 50–74 | MMG: 54-56% | None | 11 |
| Hajian-Tilaki and Auladi, 2014 [ ] | Cross-sectional | Questionnaire Interview | Iran | Cluster | 500 | Women 18–65 | BSE: 38.4 CBE:25.2% MMG: 12% | Health Belief Model | 16 |
| Tavafian et al., 2009 [ ] | Cross-sectional | Questionnaire | Iran | Cluster | 240 | Women ≥ 30 | BSE: 31.7% | Health Belief Model | 15 |
| Ahmadian et al., 2016 [ ] | Cross-sectional | Questionnaire | Malaysia | Multistage Cluster Random | 842 | Women 17–52 | BSE: NOT REPORTED | Health Belief Model | 15 |
| Jin et al., 2019 [ ] | Cross-sectional | Questionnaire | USA | Purposive | 303 | Women 50–80 | MMG: 73.3% | Andersen’s Behavioral Model | 14 |
| Charkazi et al., 2013 [ ] | Cross-sectional | Questionnaire | Iran | Cluster | 1080 | Women 30–82 | BSE: 13.1% CBE: 2.5% MMG:0.9% | Health Belief Model | 13 |
| Marmarà et al., 2017 [ ] | Cross sectional | Questionnaire | Malta | Stratified Random | 404 | Women 50–60 | MMG: NOT REPORTED | Health Belief Model & Common-Sense Model | 16 |
| Kangmennaang et al., 2019 [ ] | Cross-sectional | Questionnaire Interview | Namibia | Cluster | 9176 | Women 15–64 | BSE:35% | Health Belief Model | 14 |
| Secginli and Nahcivan, 2006 [ ] | Cross-sectional | Questionnaire | Turkey | Convenience | 656 | Women ≥ 20 | BSE: 17% MMG:25% | Health Belief Model | 13 |
| Racine et al., 2022 [ ] | Cross-sectional | Questionnaire | Canada | Convenience | 75 | Women ≥ 18 | BSE: 32% CBE: 12% MMG: 6.7% | Health Belief Model | 14 |
| Ma et al., 2012 [ ] | Cross-sectional | Questionnaire | USA | Cluster Random & Proportional | 682 | Women ≥ 40 | MMG: 50.04% | Sociocultural Health Behavior Model | 13 |
| Shakor et al., 2019 [ ] | Cross-sectional | Questionnaire | Iraq | Non-Probability (Purposive) | 750 | Women ≥ 20 | BSE: 18.0% | Health Belief Model | 15 |
| Thomas et al., 2011 [ ] | Qualitative | Interview | Iran | Quota | 31 | Women 35–65 | BCS: Not Reported | None | 11 |
| Hassan et al., 2017 [ ] | Cross-sectional | Questionnaire Interview | Egypt | Systematic Random | 600 | Women ≥ 20 | BSE: 0.3% MMG: 0.3% | None | 12 |
| Khazaee-pool et al., 2014 [ ] | Qualitative | Interview | Iran | Purposive | 16 | Women ≥ 30 | MMG: Not Reported | None | 12 |
| Moghaddam et al., 2019 [ ] | Cross-sectional | Questionnaire | Iran | Multi-Stage Random | 192 | Women ≥ 30 | BSE: 14% CBE:22.9% MMG: 10.1% | Pen-3 Model | 14 |
| Çam and Gümüs, 2009 [ ] | Cross-sectional | Questionnaire | Turkey | Stratified Random | 382 | Women ≥ 40 | BSE: 59.4% CBE:14.1% MMG: 34% | Health Belief Model | 14 |
| Moh Myint et al., 2020 [ ] | Qualitative | Interview | Myanmar | Purposive | 8 | Women 20–45 | BSE: Not Reported | None | 11 |
| Donnelly et al., 2017 [ ] | Qualitative | Interview | Qatar | Purposive | 50 | Men 30–55 | BCS: Not Reported | Ecological Perspective Klein Man’s Explanatory Model | 13 |
| Abeje et al., 2019 [ ] | Cross-sectional | Questionnaire | Ethiopia | Multi-Stage Random | 633 | Women 20–49 | BSE: 24.3% CBE:7.6% MMG: 3.8% | None | 11 |
| Carey and El-Zaemey, 2020 [ ] | Cross-sectional | Questionnaire | Australia | Simple Random | 1705 | Women ≥ 40 | MMG: 88.1% | None | 11 |
| Parsa and Kandiah, 2010 [ ] | Cross-sectional | Questionnaire | Malaysia | Multi-Stage Random | 425 | Women 23–56 | CBE:25% MMG: 13.6% | Health Belief Model | 14 |
| Tabrizi et al., 2018 [ ] | Cross-sectional | Questionnaire | Iran | Multi-Stage Random | 348 | Women 30–60 | MMG: 12% | None | 12 |
| Schoofs et al., 2017 [ ] | Cross-sectional | Questionnaire | Belgium | Quota | 350 | Women 50–69 | MMG: 81.5% | None | 11 |
| Lagerlund et al., 2015 [ ] | cohort | Questionnaire | Sweden | Simple Random | 11 409 | Women 40–74 | MMG: 88–95% | None | 14 |
| Elewonibi and BeLue, 2019 [ ] | Qualitative | Interview | Nigeria | Convenience | 94 | Women ≥ 18 | BCS: Not Reported | Pen-3 Model | 12 |
Abbreviations: BCS Breast cancer screening, BSE Breast self-examination, CBE Clinical breast examination, MMG Mammography, MQS Methodological quality score
Factors associated with BCS behaviors
The question of “What factors impact BCS behaviors in women worldwide?” is comprehensively answered through the analysis presented in Tables 4 , 5 and 6 . These tables delineate the factors influencing BSE, CBE, and MMG, respectively, as identified in the 34 reviewed articles.
Identified factors associated with breast self-examination behaviors among women around the world in the 34 reviewed articles
| Age | [ , , , , ] | [ ] | |||
| Education | [ , , – , , , ] | [ ] | [ ] | ||
| Employment status | [ , , , ] | [ ] | |||
| Income | [ , , ] | ||||
| Marital status | [ ] | ||||
| Number of Children | [ ] | ||||
| Ethnicity | None | ||||
| Region of Residence | [ ] | ||||
| Race | None | ||||
| Spouse Demographic Characteristics | [ , ] | ||||
| Hormone Therapy and History of Infertility | None | ||||
| Family History of Breast Cancer | [ , , , ] | ||||
| Personal History of Cancer or Past Breast Disorders | [ , ] | ||||
| Knowledge About Breast Cancer Screening | [ , , , , , ] | [ , , , ] | |||
| Knowledge About Breast Cancer | [ , , ] | [ , ] | |||
| Perceived Health Status | [ ] | ||||
| Attitude Towards Breast Cancer Screening | [ , ] | ||||
| Perceived Barriers | [ , , , – , , ] | [ ] | [ , ] | ||
| Perceived Benefits | [ , , , , , ] | ||||
| Self-efficacy | [ , , , , , , , ] | [ ] | |||
| Perceived Severity | [ , ] | [ , , , , ] | [ ] | ||
| Perceived Susceptibility | [ , ] | [ , , ] | |||
| Fatalistic /Religious Beliefs | [ ] | [ ] | |||
| Cultural Differences) Longer Migration Time- speaking English Well- Cultural Support) | [ ] | ||||
| Gender of the Doctor Performing the Clinical Checkup/Examinations | [ ] | ||||
| Traditional/Alternative Care | [ ] | ||||
| Social Stigma | [ ] | ||||
| Breast Cancer Screening by Family Members and Friends | None | ||||
| Hearing About BC and BCS from Health Team or in the Media | [ , ] | [ ] | |||
| Similarly, Reminder Letters, Phone Calls, or Text Messages | None | ||||
| High Level of Hope and Health Motivation for the Future | [ , , , ] | [ ] | |||
| Having Regular Checkups | [ ] | ||||
| Smoking | [ ] | ||||
| Alcohol Abstinence | None | ||||
| Physical Activity | None | ||||
| Following Healthy Diet | None | ||||
| Body Mass Index | [ ] | ||||
| Health Insurance Coverage | [ , ] | [ ] | |||
| Health Workers and Family Members Support | [ ] | [ , , ] | |||
| Access to Screening Centers | [ ] | [ ] | |||
Identified factors associated with clinical breast examination behaviors among women around the world in the 34 reviewed articles
| Clinical breast examination behaviors | |||||
|---|---|---|---|---|---|
| Age | [ , , ] | [ ] | |||
| Education | [ , , ] | [ ] | [ ] | ||
| Employment status | [ , ] | ||||
| Income | [ , , ] | ||||
| Marital status | None | ||||
| Number of Children | None | ||||
| Ethnicity | None | ||||
| Region of Residence | None | ||||
| Race | None | ||||
| Spouse Demographic Characteristics | [ ] | [ ] | |||
| Hormone Therapy and History of Infertility | [ ] | ||||
| Family History of Breast Cancer | [ , ] | [ ] | |||
| Personal History of Cancer or Past Breast Disorders | [ , ] | ||||
| Knowledge About Breast Cancer Screening | [ , , , , , ] | [ , , ] | |||
| Knowledge About Breast Cancer | [ , ] | [ ] | |||
| Perceived Health Status | None | ||||
| Attitude Towards Breast Cancer Screening | [ , ] | ||||
| Perceived Barriers | [ , ] | [ ] | [ ] | ||
| Perceived Benefits | [ , , ] | ||||
| Self-efficacy | [ ] | [ ] | |||
| Perceived Severity | [ , ] | [ ] | |||
| Perceived Susceptibility | [ , ] | [ ] | |||
| Fatalistic /Religious Beliefs | [ ] | [ ] | [ ] | ||
| Cultural Differences) Longer Migration Time- speaking English Well- Cultural Support) | [ ] | [ ] | |||
| Gender of the Doctor Performing the Clinical Checkup/Examinations | [ ] | ||||
| Traditional/Alternative Care | [ ] | ||||
| Social Stigma | [ ] | ||||
| Breast Cancer Screening by Family Members and Friends | None | ||||
| Hearing About BC and BCS from Health Team or in the Media | [ ] | ||||
| Similarly, Reminder Letters, Phone Calls, or Text Messages | None | ||||
| High Level of Hope and Health Motivation for the Future | [ , ] | [ ] | |||
| Having Regular Checkups | [ , ] | ||||
| Smoking | None | ||||
| Alcohol Abstinence | None | ||||
| Physical Activity | None | ||||
| Following Healthy Diet | None | ||||
| Body Mass Index | [ ] | ||||
| Health Insurance Coverage | [ , ] | ||||
| Health Workers and Family Members Support | [ ] | [ , , ] | |||
| Access to Screening Centers | [ ] | [ ] | |||
Identified factors associated with mammography behaviors among women around the world in the 34 reviewed articles
| Category | Factor | Mammography behaviors | |||
|---|---|---|---|---|---|
| Studies displaying a positive association | Studies displaying a negative association | Studies displaying no association | Qualitative studies | ||
| Age | [ , , ] | [ ] | |||
| Education | [ , , , , ] | [ ] | [ ] | [ ] | |
| employment status | [ , ] | [ ] | [ , , ] | ||
| Income | [ , , ] | [ , ] | |||
| Marital status | [ , ] | [ , ] | |||
| Number of Children | [ , , ] | [ ] | |||
| Ethnicity | [ , ] | ||||
| Region of Residence | [ ] | ||||
| Race | [ ] | ||||
| Spouse Demographic Characteristics | [ , ] | ||||
| Hormone Therapy and History of Infertility | [ ] | ||||
| Family History of Breast Cancer | [ , , , , , ] | [ ] | |||
| Personal History of Cancer or Past Breast Disorders | [ , , , ] | [ ] | |||
| Knowledge About Breast Cancer Screening | [ , , , , ] | [ , , , ] | |||
| Knowledge About Breast Cancer | [ , , , ] | [ ] | |||
| Perceived Health Status | [ , ] | ||||
| Attitude Towards Breast Cancer Screening | [ ] | ||||
| Perceived Barriers | [ , , , , , ] | [ ] | [ ] | ||
| Perceived Benefits | [ ] | ||||
| Self-efficacy | [ , ] | [ ] | |||
| Perceived Severity | [ ] | [ , , ] | [ ] | ||
| Perceived Susceptibility | [ , , , , ] | [ ] | |||
| Fatalistic /Religious Beliefs | [ ] | [ ] | [ , ] | ||
| Cultural Differences) Longer Migration Time- speaking English Well- Cultural Support) | [ ] | [ ] | |||
| Gender of the Doctor Performing the Clinical Checkup/Examinations | [ ] | ||||
| Traditional/Alternative Care | [ ] | ||||
| Social Stigma and Anticipated Negative | None | ||||
| Breast Cancer Screening by Family Members and Friends | [ , ] | ||||
| Hearing About BC and BCS from Health Team or in the Media | [ , , ] | [ ] | |||
| Similarly, Reminder Letters, Phone Calls, or Text Messages | [ ] | ||||
| High Level of Hope and Health Motivation for the Future | [ ] | [ , ] | |||
| Having Regular Checkups | [ , , , ] | ||||
| Smoking | [ ] | [ ] | |||
| Alcohol Abstinence | [ ] | [ ] | |||
| Physical Activity | [ ] | ||||
| Following Healthy Diet | [ , ] | ||||
| Body Mass Index | [ ] | [ , ] | |||
| Health Insurance Coverage | [ , , ] | [ , ] | |||
| Health Workers and Family Members Support | [ , , ] | [ , , , ] | |||
| Access to Screening Centers | [ , , ] | [ ] | [ ] | ||
The factors identified are categorized into nine key areas:
- Socio-demographic Factors: This includes age, education level, income, marital status, and employment status, highlighting how these variables influence screening behaviors.
- Health History: Past health experiences, family history of breast cancer, and personal health beliefs play a significant role in an individual’s decision to undergo screening.
- Knowledge: The awareness and understanding of breast cancer and the benefits of early detection through screening methods.
- Perceptions: Women’s beliefs and attitudes towards breast cancer risk, the effectiveness of screening, and the healthcare system’s role in cancer detection.
- Cultural Factors: How cultural beliefs, norms, and societal expectations shape attitudes towards breast health and screening practices.
- Cues to Action: External prompts, such as recommendations from healthcare professionals, health campaigns, or peers’ experiences, encourage women to seek screening.
- Motivation: The intrinsic and extrinsic motivators drive women to participate in screening activities.
- Self-care: The degree to which women prioritize their health and well-being, including the proactive pursuit of health screenings.
- Social Support: The influence of family, friends, and community networks in supporting or hindering screening behaviors.
The primary goal of this study was to identify the universal factors influencing BCS behaviors among women globally. Although most countries offer BCS programs [ 17 ], the nature and implementation of these programs vary significantly across different health systems and populations [ 49 ]. Consequently, the BCS methods examined in this review varied, reflecting these disparities. MMG, recognized for its efficacy in clinical studies, is predominantly used in developed countries due to its higher costs [ 8 ]. Conversely, in developing countries, BSE stands out as a widely adopted, cost-effective method for early detection [ 50 ].
Moreover, the rates of screening methods reported in the literature show considerable international variation. Countries like Sweden, Belgium, the USA, and Australia report high MMG screening rates [ 14 – 16 , 43 ], whereas BSE is more prevalent in countries like Egypt, Ethiopia, Turkey, Iran, and Iraq [ 12 , 13 , 25 , 26 , 32 ], often falling below the WHO’s recommended screening rates [ 49 ].
The WHO underscores the importance of high participation rates in screening programs to enhance their effectiveness [ 49 ]. Understanding the factors influencing participation enables health systems to adopt comprehensive strategies for prevention, early diagnosis, and BCS promotion.
Over half of the studies reviewed focused on socio-demographic factors as determinants of screening behaviors, identified in previous research as facilitators and barriers [ 51 , 52 ]. Findings indicate that demographic variables such as age, education level, income, and employment status significantly influence screening rates.
While socio-demographic status is recognized as a crucial determinant of access to BCS in both high-income [ 51 , 52 ] and middle-income countries [ 10 , 17 ], studies in European countries with organized screening programs report no correlation between screening participation and socio-demographic variables [ 53 ]. A 2011 study exploring the impact of socioeconomic inequalities on screening participation highlighted that such disparities exist even without financial barriers [ 54 ]. These variations necessitate careful interpretation, considering women’s diverse challenges in accessing screening services worldwide, including geographical, economic, and cultural obstacles.
For instance, despite Qatar’s provision of comprehensive medical services at no cost, including BCS, cultural barriers have led to only a third of eligible women utilizing these services [ 34 ]. Thus, offering organized screening programs with equitable access could gradually mitigate socioeconomic disparities.
The review also highlights that beyond a family history of breast cancer and personal breast health issues, fertility-related challenges, such as infertility and hormonal imbalances, influence screening behaviors. This finding aligns with systematic reviews from China and the USA, which examined screening factors among different populations [ 55 , 56 ]. Women with personal or familial health histories may perceive a higher susceptibility to breast cancer, thereby increasing their utilization of healthcare services for screening and diagnostic tests. This heightened awareness and concern about breast cancer risk can motivate women to adopt preventive measures, including screening. However, it is notable that many women may not pursue screening until symptomatic or following the discovery of breast cancer in close relatives [ 57 , 58 ].
The findings of the study reveal that women with comprehensive knowledge about breast cancer risk factors, symptoms, and screening methods are more likely to participate in screening programs. Conversely, women who have not undergone screening often lack awareness or believe that once screened, repeat screenings are unnecessary [ 59 ]. This lack of knowledge has been identified as a critical barrier to screening participation among Iranian and Asian women and as a predictive factor for the late diagnosis of breast cancer in Canada [ 10 , 60 , 61 ]. However, Schlueter’s study found no correlation between the level of knowledge and screening behaviors [ 62 ], indicating the complexity of this relationship.
Educational interventions targeting breast cancer awareness and screening guidelines are crucial for improving women’s knowledge and participation rates.
Perceptual factors significantly influence screening behaviors, including fewer perceived barriers and higher self-efficacy. A Chinese study highlighted reduced perceived barriers as a predictive factor for screening participation [ 55 ]. Main barriers identified include fear [ 34 , 42 , 46 , 48 ], anxiety [ 29 , 30 ], worry [ 22 , 63 ], religious beliefs and fatalism [ 32 , 46 , 48 ], financial constraints [ 34 ], language barriers [ 29 , 39 , 40 ], and embarrassment [ 63 ]. Although fear can motivate screening behavior in some contexts [ 56 ], it is predominantly an emotional barrier in the findings.
Types of fear recognized include the fear of mastectomy, diagnosis of cancer, and stigmatization [ 34 , 46 , 48 ]. Consedine et al. noted that while fear of cancer could facilitate screening, specific fears—such as those associated with medical procedures or diagnosis—often deter women from participating [ 64 ]. A meta-analysis further linked fear of breast cancer to screening behaviors [ 65 ], suggesting that mitigating fear through education and positive screening experiences could enhance participation rates.
Cultural factors, particularly religious beliefs, and fatalism, notably impact screening behaviors. Some Muslim women believe BCS is unnecessary, viewing cancer as a divine challenge or part of destiny [ 63 ]. This fatalistic view, a belief in the health locus of control being external (chance or divine will), can lead to passive health behaviors [ 66 ]. While some studies show no significant impact of religious beliefs on screening behaviors [ 67 ], the intertwined nature of these beliefs with culture and religion necessitates nuanced interventions.
Effective strategies might involve integrating breast cancer awareness and early diagnosis information within the framework of existing belief systems leveraging religious leaders to promote health messages aligned with spiritual teachings. Such approaches, using religious and spiritual elements in health messaging, have been shown to encourage screening behaviors among women [ 11 ].
The results of this review highlight that women are more likely to engage in BCS behaviors when they receive information from healthcare teams, social media, or other sources compared to those who do not consult with healthcare professionals or use social media for health information. Jones et al. emphasized that recommendations and reminders from healthcare providers are among the most effective means of directing women toward MMG and other screening tests [ 68 ]. A 2019 study further showed that ignoring cues to action, such as letters, messages, and reminder calls, correlates with lower MMG participation rates [ 69 ].
In the modern era, widespread access to information through digital media, advancements in technology, and the introduction of electronic health tools have facilitated the use of these platforms in cancer screening campaigns. For instance, smartphone applications that remind users about screening schedules and provide preventive advice through text, images, and videos represent an innovative approach to enhancing screening participation.
This review also underscores a significant link between motivation and BCS behaviors. Khazaee-Pool et al. found that motivational solid factors, such as valuing life and health responsibility, significantly encourage screening participation [ 21 ]. Moreover, studies among diverse racial and ethnic groups have identified a clear association between motivation and increased screening activities [ 70 ].
Various socio-psychological barriers, including attitudes, cultural beliefs, and communication issues, have been identified as impediments to motivation [ 71 ]. Factors contributing to low motivation for MMG include the perceived unimportance of testing, lack of support, time constraints, cost concerns, familial obligations, and a busy lifestyle [ 48 ]. Therefore, interventions aimed at enhancing motivational self-efficacy could significantly improve screening participation.
As part of self-care practices, regular health check-ups have been shown to predict screening behaviors. Reviews have highlighted a correlation between infrequent mammograms and breast exams among Asian and Korean-American women with irregular gynecological visits [ 51 , 59 ]. Although MMG can be performed without direct referrals in some countries [ 59 ], the lack of commitment to regular check-ups remains a barrier. As Pasket et al. reported, while 75% of women acknowledged the importance of periodic exams, 67% indicated that their physicians did not actively encourage MMG [ 72 ].
Improving knowledge about self-care and self-regulation is crucial for fostering regular health examination habits. The health system’s role in scheduling periodic health assessments and encouraging adherence is also vital, as demonstrated by research from the Netherlands, which linked pre-scheduled appointments and proactive general practitioner involvement to higher screening rates [ 49 ].
Regarding social support, assistance from healthcare teams and family members significantly influences screening behaviors. Lack of partner support and fear of familial disruption post-diagnosis have been noted as significant barriers among African-American women [ 68 ]. Support from family and friends, providing both financial and emotional backing, can bolster confidence, reduce fear, and encourage screening participation [ 21 , 59 , 73 ].
The review also points out that women’s financial independence and employment status in certain regions are critical in health decision-making. Conversely, many women rely on male family members to make health decisions, a group that requires targeted support from health teams for emotional, instrumental, and informational needs. Leong et al. found that social support not only reduces depression but also promotes healthier behaviors [ 74 ]. Thus, establishing support networks and self-help groups can enhance women’s knowledge, experience, and motivation regarding BCS, ultimately fostering a community of mutual encouragement and support.
This systematic review meticulously evaluated the quality of included studies to ensure their reliability and relevance. A unique aspect of the analysis is the consideration of men’s attitudes and perceptions toward BCS, acknowledging the influence of gender dynamics on screening behaviors. A comprehensive approach was undertaken, analyzing factors affecting BCS behaviors across quantitative and qualitative studies and categorizing them based on their impact on three distinct screening behaviors: BSE, CBE, and MMG. This nuanced categorization provides a detailed understanding of the diverse influences on BCS practices.
Limitations
This research was confined to online studies, potentially overlooking valuable research indexed in databases such as PubMed, Scopus, Embase, and Google Scholar or available only in print. The restriction to English-language publications may have excluded pertinent non-English studies, introducing language bias. The review’s predominance of cross-sectional studies limits the ability to ascertain causal relationships between the factors studied and screening behaviors. Additionally, the reliance on self-reported data raises concerns about the accuracy of the findings, given the potential for recall bias or the desire of participants to present themselves in a socially desirable light.
The heterogeneity of the included studies—in terms of study design, geographic location, methodological approach, demographic characteristics, sample size, screening methods employed, and the intervals between screenings—complicates direct comparisons and may affect the generalizability of the findings.
This systematic review synthesizes a broad array of research on the factors influencing BCS behaviors among women worldwide. By examining various screening methods and participation rates, along with identifying determinants of screening behavior, this study contributes valuable insights to the field of public health. The findings highlight the complex interplay of factors affecting screening behaviors and provide evidence-based guidance for policymakers and health promotion professionals. This knowledge is crucial for developing targeted interventions that can effectively encourage BCS practices, ultimately contributing to breast cancer prevention and early detection.
Acknowledgements
We thank the Isfahan University of Medical Sciences and the Isfahan School of Health for their support. Our thanks also go to all who contributed to the conceptualization, execution, and analysis of this work.
Abbreviations
| BCS | Breast cancer screening |
| BSE | Breast self-examination |
| CBE | Clinical breast examination |
| MMG | Mammography |
| MQS | Methodological quality score |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| WHO | World Health Organization |
Authors’ contributions
B.T. and H.S. conceived the project; B.T. and H.S. performed the literature search; all authors contributed to the literature analysis and synthesis of data; F.Z. and A.F. created the figures and tables; B.T. and H.S. wrote the review. All authors were involved in further editing and finalizing the manuscript.
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
Declarations.
This manuscript received ethical approval from the Isfahan University of Medical Sciences (Science code: 3400585; Ethical Code: IR.MUI.RESEARCH.REC.1400.343). Given the nature of this systematic review, consent to participate was not applicable.
Not applicable.
The authors declare no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- Open access
- Published: 09 July 2024
Implementation of social needs screening for minoritized patients newly diagnosed with breast cancer: a mixed methods evaluation in a pragmatic patient navigation trial
- Stephenie C. Lemon 1 ,
- Amy M. LeClair 2 ,
- Erika Christenson 3 ,
- Deborah Amburgey 3 ,
- Madyson FitzGerald 3 ,
- Howard Cabral 4 ,
- Chris Lloyd-Travaglini 4 ,
- Cheryl R. Clark 5 ,
- Feng Qing Wang 2 ,
- Joellen Ross 6 ,
- Ellen Ohrenberger 6 ,
- Jennifer S. Haas 7 ,
- Karen N. Freund 2 ,
- Tracy A. Battaglia 3 &
the TRIP Consortium [representative Tracy A. Battaglia
BMC Health Services Research volume 24 , Article number: 783 ( 2024 ) Cite this article
Metrics details
Social needs inhibit receipt of timely medical care. Social needs screening is a vital part of comprehensive cancer care, and patient navigators are well-positioned to screen for and address social needs. This mixed methods project describes social needs screening implementation in a prospective pragmatic patient navigation intervention trial for minoritized women newly diagnosed with breast cancer.
Translating Research Into Practice (TRIP) was conducted at five cancer care sites in Boston, MA from 2018 to 2022. The patient navigation intervention protocol included completion of a social needs screening survey covering 9 domains (e.g., food, transportation) within 90 days of intake. We estimated the proportion of patients who received a social needs screening within 90 days of navigation intake. A multivariable log binomial regression model estimated the adjusted rate ratios (aRR) and 95% confidence intervals (CI) of patient socio-demographic characteristics and screening delivery. Key informant interviews with navigators (n = 8) and patients (n = 21) assessed screening acceptability and factors that facilitate and impede implementation. Using a convergent, parallel mixed methods approach, findings from each data source were integrated to interpret study results.
Patients’ (n = 588) mean age was 59 (SD = 13); 45% were non-Hispanic Black and 27% were Hispanic. Sixty-nine percent of patients in the navigators’ caseloads received social needs screening. Patients of non-Hispanic Black race/ethnicity (aRR = 1.25; 95% CI = 1.06–1.48) and those with Medicare insurance (aRR = 1.13; 95% CI = 1.04–1.23) were more likely to be screened. Screening was universally acceptable to navigators and generally acceptable to patients. Systems-based supports for improving implementation were identified.
Conclusions
Social needs screening was acceptable, yet with modest implementation. Continued systems-based efforts to integrate social needs screening in medical care are needed.
Peer Review reports
Contributions to the literature
This study observed high degrees of acceptability of social needs screening delivery by patient navigators and high degree of acceptability of social needs screening by patients in the context of treatment for breast cancer among newly diagnosed, minoritized women.
This study observed that despite resources and training, screening for social needs by patient navigators in the context of routine cancer is delivered to modest percentage (69%) of eligible patients.
This study identified systems-level interventions that could enhance performance of social needs screening by patient navigators in breast cancer care.
Despite decades of progress in breast cancer treatment, disparities in outcomes persist [ 1 , 2 ]. Women diagnosed with breast cancer who are Black, Hispanic, speak a primary language other than English, and/or who have inadequate insurance experience high rates of delays in care, contributing to increased morbidity and mortality [ 2 , 3 , 4 , 5 ].
Unmet social needs such as housing insecurity, food insecurity and lack of transportation are barriers to timely cancer care. An estimated 20% of patients with cancer in the United States experience at least one unmet social need, with higher rates among individuals of Black race, Hispanic ethnicity and/or low socioeconomic status [ 6 , 7 , 8 ]. Calls for the integration of systematic social needs screening with appropriate follow-up into medical care in general and cancer care specifically have accelerated as a means to ameliorate barriers and improve equitable health outcomes [ 9 , 10 , 11 , 12 , 13 ].
However, systematic social needs screening remains aspirational, as it not a routine part of cancer care in most delivery sites [ 14 ]. Little research has assessed how to successfully implement social needs screening in the context of cancer care. Patient navigators are vital members of cancer care teams who are potentially well-suited to deliver social needs screenings and work with patients to address identified needs. Patient navigation is an evidence-based approach for reducing cancer-related disparities, now considered standard of care [ 15 , 16 , 17 ]. Patient navigators are typically responsible for working with patients, especially those at high risk for disparities, to identify and overcome barriers to receipt of timely, high quality cancer care [ 18 , 19 ]. Thus, screening for and addressing social needs among at-risk patients is consistent with the goals and values of patient navigation.
The purpose of this study is to gain an in-depth understanding of the implementation of social needs screening by patient navigators for newly diagnosed patients with breast cancer from minoritized backgrounds in a pragmatic trial. Specifically, the aims are to quantify the percentage of eligible patients who received social needs screening and patients factors related to delivery of social needs screening and to describe the acceptability of social needs screening and factors that could improve the implementation from the patient navigator and patient perspectives.
The Translating Research Into Practice (TRIP) study
The aim of this mixed methods project was to describe social needs screening implementation in a pragmatic patient navigation intervention trial for minoritized women newly diagnosed with breast cancer. Data are from the TRIP study [ 20 ]. Briefly, TRIP used a community engaged approach, with academic partners from four Clinical and Translational Science Institutes in Massachusetts partnering with the Boston Breast Cancer Equity Coalition to address disparities in breast cancer outcomes. TRIP was a Type 1 hybrid effectiveness-implementation trial [ 21 ] that utilized a cluster-randomized, stepped wedge design [ 22 , 23 ], and was conducted in five cancer care centers in Boston, MA between 2018 to 2022.
TRIP aimed to improve receipt of timely, quality breast cancer care through implementation of an integrated, evidence-based patient navigation intervention. The TRIP intervention was integrated as standard practice at the participating sites, each of which had existing breast cancer patient navigation programs [ 24 ]. The intervention entailed patient navigators using a standardized, 11-step protocol [ 25 ], which included social needs screening and referrals, and was grounded in the principles of patient navigation and care management [ 26 ]. The work of the patient navigators was supported by a project-specific shared REDCap registry that guided their caseload through each of the 11-steps. Initial training in administering the TRIP protocol involved in-person and online training in the protocol, use of the registry and use of the standardized social needs screening tools and referral platforms. Quarterly case-based navigator network meetings created a learning collaborative for navigators to share best practices in protocol implementation. In response to navigator’s request for support in asking sensitive questions, a one-hour training in principles of empathic inquiry and using patient-centered approaches to screening adapted from an existing program that integrates basic principles of motivational interviewing and trauma-informed care [ 27 ] was conducted after the project began.
Social needs screening delivery
Study design.
Data were from the larger TRIP study trial, which utilized a prospective, stepped-wedge design. For this analysis, given the focus on delivery of social needs screening at a single time point, we used a cross-sectional analysis to quantify the delivery of social needs screening per study protocol, defined as completion within 90 days of intake.
Participants
To be included in the study population, women had to have a new breast cancer diagnosis between June, 2018 and August, 2021, be at least 18 years of age, and live within the Great Boston area and meet at least one of the following criteria: Black race and/or Hispanic ethnicity, speak a primary language other than English, have public insurance or be uninsured at the time of diagnosis and be part of the navigators’ caseload, as indicated by being entered into the project registry.
Social needs screening
Social needs screening was a core component of the TRIP intervention. A standardized screening tool was used at each site with a navigation protocol requiring administration at intake and three-month follow-up intervals. A study-specific tool was adapted from published web-based screening tools and platforms (i.e., Findhelp [ 28 ], THRIVE [ 29 ]. The tool assessed 9 social need domains: housing insecurity, food insecurity, transportation, paying for treatment, paying for basic utilities, employment, education, family caregiving and legal issues. hese data were prospectively entered into either the site’s Epic Electronic Health Record or the Aunt Bertha/FindHelp platform. For this paper, the intake social needs screening was assessed. The study protocol required eligible patients to receive a social needs screening within 90 days of intake. Data were abstracted from Epic or the Aunt Bertha/FindHelp platform for analysis.
Patient socio-demographic characteristics
Patient socio-demographic characteristics were obtained from each site’s Cancer Registry and electronic health record. Characteristics assessed included patient age, race/ethnicity, preferred language and insurance status as indicated at time of diagnosis.
Statistical analysis
Frequency distributions quantified delivery of a social needs screening. Bivariate analyses were conducted to assess the frequency of screening for social needs according to patient socio-demographic characteristics. Log binomial regression models were computed to estimate the rate ratio of each patient socio-demographic characteristic with delivery of social needs screening, accounting for clustering within cancer care site. Single variable models and a multivariable model which included all patient socio-demographic characteristics were computed. A two-sided p-value of < 0.05 was used as a threshold of statistical significance. Analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC).
Acceptability of social needs screening by patient navigators & patients
Key informant interviews were conducted with the participating patient navigators and a sample of patients. Patient navigator interviews were conducted 27 months after the TRIP protocol was first introduced at the respective project site. Patient interviews were conducted between February and April of 2022. These one-time interviews were conducted by trained project staff over the telephone. The interviews included questions about all of the components of the TRIP intervention: This analysis focuses on the social needs screening component.
Patient navigators who participated in the interviews were individuals who were employed by the partnering cancer care centers and working on the TRIP protocol at the time of the respective interviews. All interviews were audio recorded and followed a semi-structured interview guide. Patient navigators were compensated $50. A total of eight patient navigators were eligible and eight participated. Two of the eight were nurse navigators and six were patient navigators without clinical backgrounds.
Patients who participated in the interviews: 1) were a patient during the last 6 month of study enrollment (5/1/2021–11/30/2021), 2) spoke English as their preferred language, and 3) had at least one completed social needs assessment. An opt-out letter was sent to all identified patients; patients who did not opt-out received a call from study staff one week after the mailing with up to two follow-up calls. All interviews conducted over the phone by trained study team members, were audio recorded, and followed a semi-structured interview guide. Patients were compensated $25 for their participation. A total of 21 patients were interviewed, with representation from each site.
Interview guides
Semi-structured interview guides were developed by the project team and are available in a supplementary file. The guide for patient navigators was designed to understand their experiences and perspectives related to the TRIP navigation protocol and their experiences in the project overall. Open-ended questions with prompts were designed to elicit perspectives related to administering the social needs screening to patients, with an emphasis on understanding acceptability of conducting the screening and barriers and facilitators to implementing the screening tool. [ 30 ] The guide for patients was designed to understand their experiences and perspectives related to receiving patient navigation services. Specific questions with prompts were designed to elicit perspectives related to being administered the social needs screening and any subsequent referrals or other follow-up received based on the screening, with an emphasis on acceptability and associated barriers and facilitators [ 30 ].
Qualitative analysis
The interview recordings were transcribed by Datagain. Transcripts were de-identified and reviewed against audio recordings for accuracy. Rapid qualitative analysis was used [ 31 ] to synthesize interview findings. Rapid methods are increasingly used in implementation science and have demonstrated a high degree of concordance with traditional qualitative analytic techniques. Rapid qualitative analysis is especially useful when analyzing action-oriented, time-sensitive qualitative research [ 31 , 32 ]. Following a previously established approach [ 33 , 34 ], TRIP team members created a template of domains based on the interview guide that was used to summarize each interview. Two lead team members summarized the first interview independently and discussed their findings to develop a summary matrix and ensure consistency and completeness. The remaining transcripts were divided among four other team members for categorizing (DA, VX, MA, TZ). Team members reviewed each other’s completed summary templates, and made refinements to the template and process as needed. The two team leads with extensive experience in qualitative research (AML, EC) served as the secondary reviewer for each transcript. The summary templates were then combined to create a matrix of responses across all interviews. From this matrix, the domains were reviewed and synthesized into resulting content areas, themes, and representative quotations. Patient navigator and patient interviews were analyzed separately. Results were then compared across interview type to determine commonalities and differences and draw overall inferences.
Mixed methods data integration
This study used a convergent parallel mixed methods approach to integrate and interpret the results across the three study sources: quantitative social screenings delivery data, patient navigator interviews and patient interviews. In this approach, data are collected concurrently, analyzed separately and given equal priority. Data are then integrated/compared to interpret study results. After analyses were completed, the data from all sources were compared and integrated. Through a series of meetings, the study team examined the relationship between the data from the three sources (quantitative, 2 qualitative sources) to create an explanatory model for the modest findings related to social needs screening delivery.
TRIP study sample
The TRIP intervention study sample included 588 women who received a navigator intake assessment. A description of the sample is presented in Table 1 . One-third was aged 65 or older. Almost half of the sample (47%) were of African American or Black race and more than one-fourth (27%) were Hispanic. Almost half primarily spoke a language other than English with over 20 languages reported, and had public insurance.
The overall percentage of patients screened for social needs at intake and screening rates according to patient socio-demographic characteristics are presented in Table 1 . Overall, 69% of patients in the navigators’ caseloads received this screening. Differences in rate of screening by site were observed, ranging from 40 to 100%. Results were similar across the bivariate and multivariable models (Table 2 ). In the multivariable model, patients of non-Hispanic Black race/ethnicity were more like to receive social needs screening (aRR = 1.25; 95% CI = 1.06–1.48) compared to patients who were non-Hispanic white. Patients with Medicare insurance were more likely to be screened (aRR = 1.13; 95% CI = 1.04–1.23), compared to patients with private insurance.
Patient navigator and patient acceptability of social needs screening
Table 3 describes acceptability-related themes and representative quotations from patient navigators and patients. Patient navigators found screening patients for social needs to be unanimously acceptable. They strongly believed in the purpose and goals of screening patients for social needs in the context of their broader cancer care. The patient navigators additionally perceived that screening for social needs aligned with their professional values and understanding of their roles as patient navigators. The navigation protocol and tools for conducting screenings were found to be acceptable and allowed for them to approach their work in a more standardized manner.
Patients also generally found being screened for social needs to be acceptable. Many patients reported that they felt comfortable being screened for social needs. They additionally reported that being screened for social needs and receiving assistance to address them made them feel more supported during their cancer care. Some patients noted perceptions of stigma around being asked sensitive questions and the emotional impact they experienced in response to having social needs. However, these individuals also noted that the patient navigators were non-judgmental and supportive in their approach, which influenced their willingness to answer social needs questions.
Factors could improve social needs screening implementation
Table 4 presents barriers and facilitators to social needs screening. Four themes were identified that could improve social needs screening implementation. First, patient navigators expressed that being well-integrated into the larger cancer care team was an important facilitator of establishing relationships with patients. Several patient navigators reported that being in close physical proximity to the physicians and nurses increased these providers’ awareness of and trust in the patient navigators, which resulted in direct provider introductions of patients to the navigators. These “warm handoffs” facilitated more positive navigator-patient interactions, including patient acceptability of social needs screening.
Second, patient navigators indicated a strong preference for first meeting and conducting intake social needs screenings with patient’s in-person, rather than over the phone. This was perceived to help establish relationships and patient comfort level with the patient navigator, which set the stage for more fruitful phone-based follow-up conversations. Third, some patient navigators reported low levels of comfort screening and more generally serving patients who spoke different languages than they did. Lastly, patient navigators described that using empathic approaches improved their self-efficacy in delivering social needs screening. Such approaches, which allow navigators to approach screening more conversationally rather than simply reading a survey, allowed navigators to connect better with patients, which was perceived to increase patient comfort level as well.
This mixed methods study examined the implementation of social needs screening among newly diagnosed breast cancer patients at high risk for poor outcomes by patient navigators in a pragmatic trial. Social needs screening was universally acceptable by patient navigators, with patients indicating a high degree of acceptability. However, despite a standardized protocol and patient navigators with effort supported to conduct this screening, patient navigators delivered systematic social needs screening to less than 70% of eligible patients. Overall, approximately seven out of ten eligible patients received an intake social needs screening. These findings mirror results of similar efforts to implement social needs screening in primary care, emergency department, and oncology settings [ 29 , 35 , 36 , 37 ], with low or modest implementation despite supports to providers to conduct screenings.
Similar to other studies [ 38 ], our findings indicate that social needs screening is highly acceptable to providers. This investigation adds to the current literature by focusing on social needs screening in cancer care by patient navigators. Many cancer care sites employ patient navigators as part of the care team [ 24 ], and social needs screening is consistent with established goals and values of patient navigation [ 18 ]. In this study, navigators universally perceived that addressing patient social needs was important and consistent with their responsibilities. Furthermore, in the context of this research study, patient navigators appreciated the structure provided by the standardized screening tool and referral system.
We identified important differences in screening by patient socio-demographic characteristics. Non-Hispanic Black women had the highest likelihood of screening, compared to other race/ethnicities. Patients with Medicare were more likely to be screened, even when controlling for age. We explored the possibility that individuals with disabilities who receive Medicare, typically indicated by being under age 65, were more likely to be screened. We conducted an ad hoc exploratory analysis that included an interaction between insurance status and age < 65 and over 65) to determine if there was differential response across insurance categories for those younger than 65 compared to those 65 and over. We found no such differential response, hence indicating that higher degree of screening among those with Medicare insurance was not due to disability (indicated by younger age) status. Reasons for differences in screening by patient socio-demographic characteristics observed in this study require further investigation for potentially intervenable factors including implicit bias of the navigators about who may have social needs or which patients are comfortable with social needs inquiry.
Despite universal acceptability, site-level differences in screening rates were observed. The site with the lowest screening rate (40%) experienced navigator staffing shortages prior to [ 24 ] and on and off throughout the course of the project. Likewise, the two sites with the highest screening rates (97% and 100%) had established navigation programs that were engrained within the cancer care teams [ 24 ]. Previous studies have found that patient navigators can be pulled into other support tasks not associated with navigation [ 39 ], which was reported anecdotally by navigators in this project in the context of the COVID-19 pandemic. Research assessing the implementation of social needs screening in primary care settings shows that when systems are in place to support social needs screening, it can be implemented widely [ 29 ], and is acceptable to both providers, patients, and caregivers [ 40 , 41 ]. Our findings further highlight the need for cancer care sites to invest in systems that support consistency of navigation services.
Also similar to previous studies [ 38 ], our findings indicate that social needs screening is acceptable to patients. Patients in this study acknowledged the potential stigma and emotional distress that could be associated with sharing personal information on social needs with a care provider. However, they for the most part expressed comfort being asked and sharing their personal circumstances and needs with the patient navigators, because they felt like the navigators were acting in their best interest. Non-clinical patient navigators, who share similar socio-demographic backgrounds and lived experiences to the patient populations they serve, may be particularly well-positioned to establish the requisite comfort and trust in the provider-patient relationship to increase patient acceptability, and willingness to participate in the social needs screening and referral process [ 42 ]. This would be consistent with other studies that demonstrate a positive effect on outcomes when there is race and language concordance among patients and navigators [ 43 ].
Our qualitative findings additionally highlight systems-level factors that could improve social need screening implementation. Patient navigators simultaneously noted that integration into the broader cancer care team, facilitated by physical proximity, and the ability to meet and conduct initial social needs screenings with patients onsite during a clinic visit, were important for setting the foundation of comfortable, trusting relationships with patients. Previous studies have observed the importance of integrating patient navigators into the healthcare team to achieving robust and successful patient navigation programs [ 44 ], and trust building is a core principle of patient navigation [ 19 ]. Allocation of appropriate physical space in close proximity to the larger care team has been identified as a best practice for supporting non-clinical health worker integration into care teams [ 45 ]. However, patient navigators can be physically siloed from the care team, relegated to non-clinical space or telephone only contact with patients. Presence afforded by physical proximity may help legitimize patient navigators as part of the care team.
Some navigators expressed less comfort screening patients who spoke different languages than they did and concerns about patient comfort with disclosing their needs, even though no differences in delivery of screening by patient primary language were observed. We were unable to assess patient perspectives on this, as the patient interviewers were limited to English speakers. However, a breadth of prior research indicates that language and cultural concordance are associated with more trusting patient-provider relationships [ 46 ]. Further investments on the part of health care systems to identify and employ patient navigators with the ability to speak the languages that better reflect the patients they serve may be needed to increase screening, and referrals for social needs.
Patient navigators in this study reported that the ability to approach social needs screening in a manner that was conversational and engaging was key to their comfort-level. The TRIP project incorporated training in empathic inquiry based on early feedback from the patient navigators that they were uncomfortable simply reading a checklist-based survey to patients on sensitive topics and that they felt patients were less likely to answer honestly with this type of approach. The goal is to support health professionals to deliver social needs screening in a manner that is non-judgmental, collaborative, and compassionate, and uses a conversational approach. Our qualitative findings suggest that this approach was well-received and improved navigator self-efficacy. Cancer care sites should invest in communications skills training to support patient-centered care and potentially increase the impact of their investments in patient navigation programs.
Study strengths include the diverse patient sample and the mixed methods approach. However, several limitations must be acknowledged. The study focused on social needs screening and did not assess referrals for identified needs or follow-up, which are essential to address social needs and warrant future investigation. Reasons for the observed differences in social needs screening receipt by race and ethnicity require further investigation for potentially intervenable factors. However, given the convergent parallel design, we were not able to explore these results with our qualitative methods. We also could interpret findings related to women of other race because the sample size is small and the group is very heterogenous. Generalizability is limited as the study occurred in Boston, MA, which has well-established cancer care sites that do not reflect care provision in other regions. The patient interviews were limited to individuals who speak English, so patient barriers related to linguistic equity could not be assessed.
Social needs screening is becoming a standard of care not only for cancer care but for all clinical practice. The Centers for Medicare and Medicaid (CMS) have included screening for health related social needs as one of five priority areas in their framework for achieving health equity [ 13 ]. Rules on social needs screening is already part of payment models for post-acute care, and currently 32 states require social needs screening as part of their payment models within their Medicaid program [ 47 ]. The CMS 2024 Medicare Physician Fee Schedule (PFS) Final Rule,1 approved in November 2023, now allows patient navigation services and screening for social needs to be reimbursed under new codes. With this rule in place, practices nationwide will need to address findings such as ours that impede optimization of evidence-based navigation [ 48 ]. Our findings are therefore critical and timely from a policy perspective.
This study observed high rates of acceptability of social needs screening on the part of patients and patient navigators; however, despite resources and training to achieve this health equity measure, the screening delivery rate was modest. We identify systems-level interventions that could enhance performance of social needs assessment, including integration of the navigator or other staff conducting the screening into the health care team, conducting screening during in-person encounters, employing screening with language and cultural congruity with the patient populations, and empathic training to increase efficacy in delivering social needs screening.
Availability of data and materials
The quantitative datasets used and/or analyzed during this study are available from the senior author (T. Battaglia) upon reasonable request. Qualitative datasets will not be made available because doing so violates the study’s informed consent.
Abbreviations
Adjusted rate ratio
Confidence interval
Translating Research Into Practice
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48.
Article PubMed Google Scholar
Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72(6):524–41.
Ko NY, Hong S, Winn RA, Calip GS. Association of insurance status and racial disparities with the detection of early-stage breast cancer. JAMA Oncol. 2020;6(3):385–92.
Article PubMed PubMed Central Google Scholar
Balazy KE, Benitez CM, Gutkin PM, Jacobson CE, von Eyben R, Horst KC. Association between primary language, a lack of mammographic screening, and later stage breast cancer presentation. Cancer. 2019;125(12):2057–65.
Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453–63.
Raber M, Jackson A, Basen-Engquist K, Bradley C, Chambers S, Gany FM, et al. Food insecurity among people with cancer: nutritional needs as an essential component of care. J Natl Cancer Inst. 2022;114(12):1577–83.
Fan Q, Keene DE, Banegas MP, Gehlert S, Gottlieb LM, Yabroff KR, Pollack CE. Housing insecurity among patients with cancer. J Natl Cancer Inst. 2022;114(12):1584–92.
Graboyes EM, Chaiyachati KH, Sisto Gall J, Johnson W, Krishnan JA, McManus SS, et al. Addressing transportation insecurity among patients with cancer. J Natl Cancer Inst. 2022;114(12):1593–600.
Bona K, Keating NL. Addressing social determinants of health: now is the time. J Natl Cancer Inst. 2022;114(12):1561–3.
Leary JC, Rijhwani L, Bettez NM, Harrington Y, LeClair AM, Garg A, Freund KM. Parent perspectives on screening for social needs during pediatric hospitalizations. Hosp Pediatr. 2022;12(8):681–90.
Nohria R, Xiao N, Guardado R, Drainoni ML, Smith C, Nokes K, Byhoff E. Implementing health related social needs screening in an outpatient clinic. J Prim Care Community Health. 2022;13:21501319221118810.
Garg A, Toy S, Tripodis Y, Silverstein M, Freeman E. Addressing social determinants of health at well child care visits: a cluster RCT. Pediatrics. 2015;135(2):e296-304.
Centers for Medicare & Medicaid Services. [Available from: https://www.cms.gov/priorities/health-equity/minority-health/equity-programs/framework .
National Cancer Institute. Addressing Social Risks in Cancer Care [Available from: https://healthcaredelivery.cancer.gov/social-risks/ .
Nelson HD, Cantor A, Wagner J, Jungbauer R, Fu R, Kondo K, et al. Effectiveness of patient navigation to increase cancer screening in populations adversely affected by health disparities: a meta-analysis. J Gen Intern Med. 2020;35(10):3026–35.
Liu D, Schuchard H, Burston B, Yamashita T, Albert S. Interventions to reduce healthcare disparities in cancer screening among minority adults: a systematic review. J Racial Ethn Health Disparities. 2021;8(1):107–26.
Freund KM, Battaglia TA, Calhoun E, Darnell JS, Dudley DJ, Fiscella K, et al. Impact of patient navigation on timely cancer care: the Patient Navigation Research Program. J Natl Cancer Inst. 2014;106(6):dju115.
Freeman HP. The origin, evolution, and principles of patient navigation. Cancer Epidemiol Biomarkers Prev. 2012;21(10):1614–7.
Freeman HP, Rodriguez RL. History and principles of patient navigation. Cancer. 2011;117(15 Suppl):3539–42.
PubMed Google Scholar
Battaglia TA, Freund KM, Haas JS, Casanova N, Bak S, Cabral H, et al. Translating research into practice: Protocol for a community-engaged, stepped wedge randomized trial to reduce disparities in breast cancer treatment through a regional patient navigation collaborative. Contemp Clin Trials. 2020;93:106007.
Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50(3):217–26.
Hemming K, Haines TP, Chilton PJ, Girling AJ, Lilford RJ. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ. 2015;350:h391.
Article CAS PubMed Google Scholar
Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials. 2007;28(2):182–91.
LeClair AM, Battaglia TA, Casanova NL, Haas JS, Freund KM, Moy B, et al. Assessment of patient navigation programs for breast cancer patients across the city of Boston. Support Care Cancer. 2022;30(3):2435–43.
Freund KM, Haas JS, Lemon SC, Burns White K, Casanova N, Dominici LS, et al. Standardized activities for lay patient navigators in breast cancer care: Recommendations from a citywide implementation study. Cancer. 2019;125(24):4532–40.
SB; G. Principles of Health Care Management: Foundations for a Changing Health Care System: Jones and Barlett Publishers; 2011.
Oregon Primary Care Association. Empathic Inquiry. Available from: https://orpca.org/empathic-inquiry/ .
Findhelp. Connected social care for healthier communities. Available from: https://company.findhelp.com/ .
Buitron de la Vega P, Losi S, Sprague Martinez L, Bovell-Ammon A, Garg A, James T, et al. Implementing an EHR-based screening and referral system to address social determinants of health in primary care. Med Care. 2019;57 Suppl 6 Suppl 2:S133-s9.
Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38(2):65–76.
Palinkas LA, Mendon SJ, Hamilton AB. Innovations in Mixed Methods Evaluations. Annu Rev Public Health. 2019;40:423–42.
Taylor B, Henshall C, Kenyon S, Litchfield I, Greenfield S. Can rapid approaches to qualitative analysis deliver timely, valid findings to clinical leaders? A mixed methods study comparing rapid and thematic analysis. BMJ Open. 2018;8(10):e019993.
Ryan GW, Goulding M, Borg A, Minkah P, Hermann S, Fisher L, et al. Clinician perspectives on pediatric COVID-19 vaccination: A qualitative study in central and western. Massachusetts Prev Med Rep. 2022;29:101966.
Goulding M, Ryan GW, Minkah P, Borg A, Gonzalez M, Medina N, et al. Parental perceptions of the COVID-19 vaccine for 5- to 11-year-old children: Focus group findings from Worcester Massachusetts. Hum Vaccin Immunother. 2022;18(6):2120721.
Beavis AL, Sanneh A, Stone RL, Vitale M, Levinson K, Rositch AF, et al. Basic social resource needs screening in the gynecologic oncology clinic: a quality improvement initiative. Am J Obstet Gynecol. 2020;223(5):735.e1-.e14.
Fiori K, Patel M, Sanderson D, Parsons A, Hodgson S, Scholnick J, et al. From policy statement to practice: integrating social needs screening and referral assistance with community health workers in an urban academic health center. J Prim Care Community Health. 2019;10:2150132719899207.
Loo S, Anderson E, Lin JG, Smith P, Murray GF, Hong H, et al. Evaluating a social risk screening and referral program in an urban safety-net hospital emergency department. J Am Coll Emerg Physicians Open. 2023;4(1):e12883.
Brown EM, Loomba V, De Marchis E, Aceves B, Molina M, Gottlieb LM. Patient and patient caregiver perspectives on social screening: a review of the literature. J Am Board Fam Med. 2023;36(1):66–78.
Clark JA, Parker VA, Battaglia TA, Freund KM. Patterns of task and network actions performed by navigators to facilitate cancer care. Health Care Manage Rev. 2014;39(2):90–101.
De Marchis EH, Hessler D, Fichtenberg C, Adler N, Byhoff E, Cohen AJ, et al. Part I: a quantitative study of social risk screening acceptability in patients and caregivers. Am J Prev Med. 2019;57(6 Suppl 1):S25-s37.
Schickedanz A, Hamity C, Rogers A, Sharp AL, Jackson A. Clinician experiences and attitudes regarding screening for social determinants of health in a large integrated health system. Med Care. 2019;57 Suppl 6 Suppl 2(Suppl 6 2):S197-s201.
Natale-Pereira A, Enard KR, Nevarez L, Jones LA. The role of patient navigators in eliminating health disparities. Cancer. 2011;117(15 Suppl):3543–52.
Charlot M, Santana MC, Chen CA, Bak S, Heeren TC, Battaglia TA, et al. Impact of patient and navigator race and language concordance on care after cancer screening abnormalities. Cancer. 2015;121(9):1477–83.
Kokorelias KM, Shiers-Hanley JE, Rios J, Knoepfli A, Hitzig SL. Factors influencing the implementation of patient navigation programs for adults with complex needs: a scoping review of the literature. Health Serv Insights. 2021;14:11786329211033268.
PubMed PubMed Central Google Scholar
Massachusetts Association of Community Health Workers. How to Integrate CHWs. Available from: https://machw.org/employers/integrating/ .
Hsueh L, Hirsh AT, Maupomé G, Stewart JC. Patient-provider language concordance and health outcomes: a systematic review, evidence map, and research agenda. Med Care Res Rev. 2021;78(1):3–23.
Hinton E; Raphael J. 10 Things to know about medicaid managed care. Available from: https://www.kff.org/medicaid/issue-brief/10-things-to-know-about-medicaid-managed-care/ .
Bonavitacola J. Rule change for patient navigation billing is a boon to oncology care, practice leaders say. Am J Managed Care. 2024;30(2):SP100.
Google Scholar
Download references
Acknowledgements
We would like to thank the members of the TRIP consortium for their contributions to this project.
Informed Verbal consent
Informed Verbal consent was obtained from the participants. This was approved by the IRB at Boston Medical Center/Boston University.
Translating Research Into Practice (TRIP) Consortium
• Beth Israel Deaconess Medical Center (Ted A. James MD, Ellen Ohrenberger RN BSN, JoEllen Ross RN BSN, Jessica Shenkel MA).
• Boston Breast Cancer Equity Coalition Steering Committee (Susan T. Gershman MS MPH PhD CTR, Mark Kennedy MBA, Anne Levine MEd MBA, Erica T. Warner ScD MPH).
• Brigham and Women’s Hospital (Cheryl R. Clark MD ScD).
• Boston Medical Center (Tracy A. Battaglia MD MPH, Naomi Y. Ko MD, Erika Christenson, MPH, Debi Amburgey, BS, Julia Vance, BA, Madyson FitzGerald BS, Victoria Xiao, BS, Tony Zhao, MS).
• Boston University (Howard J. Cabral PhD MPH, Clara Chen MHS, Christine Lloyd-Travaglini MPH, Julianne Dugas, MS).
• Dana-Farber Cancer Institute (Magnolia Contreras MSW MBA, Rachel A. Freedman MD MPH).
• Dana-Farber/Harvard Cancer Center (Karen Burns White MS).
• Dartmouth Institute (Christine Gunn PhD).
• Massachusetts General Hospital (Beverly Moy MD, Jennifer S. Haas MD MPH, Caylin Marotta MPH, Amy J Wint MSc).
• Tufts Medical Center (Karen M. Freund MD MPH, W, Amy M. LeClair PhD MPhil, Susan K. Parsons MD MRP, Feng Qing Wang BA).
• University of Massachusetts Lowell (Serena Rajabiun MA MPH PhD).
• University of Massachusetts Chan Medical School (Stephenie C. Lemon PhD MS).
Research reported in this publication was supported by the National Center for Advancing Translational Sciences and the Office of Behavioral and Social Sciences Research of the National Institutes of Health under Award Number U01TR002070. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under the Harvard University CTSA Award Number UL1TR002541, Tufts University CTSA Award Number UL1TR002544, Boston University CTSA Award Number 1UL1TR001430, and University of Massachusetts CTSA Award Number UL1 TR001453-03. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and affiliations.
Division of Preventive and Behavioral Medicine, UMass Chan Medical School, 55 Lake Avenue North, Worcester, MA, 01655, USA
Stephenie C. Lemon
Tufts Medical Center, Boston, MA, USA
Amy M. LeClair, Feng Qing Wang & Karen N. Freund
Boston Medical Center, Boston, MA, USA
Erika Christenson, Deborah Amburgey, Madyson FitzGerald, Tracy A. Battaglia & Ted A. James
Boston University School of Public Health, Boston, MA, USA
Howard Cabral & Chris Lloyd-Travaglini
Brigham and Women’s Hospital, Boston, MA, USA
Cheryl R. Clark
Beth Israel Deaconess Medical Center, Boston, MA, USA
Joellen Ross & Ellen Ohrenberger
Massachusetts General Hospital, Boston, MA, USA
Jennifer S. Haas
You can also search for this author in PubMed Google Scholar
- Ted A. James
- , Ellen Ohrenberger
- , Joellen Ross
- , Jessica Shenkel
- , Susan T. Gershman
- , Mark Kennedy
- , Anne Levine
- , Erica T. Warner
- , Cheryl R. Clark
- , Tracy A. Battaglia
- , Naomi Y. Ko
- , Erika Christenson
- , Debi Amburgey
- , Julia Vance
- , Madyson FitzGerald
- , Victoria Xiao
- , Tony Zhao
- , Howard J. Cabral
- , Clara Chen
- , Christine Lloyd-Travaglini
- , Julianne Dugas
- , Magnolia Contreras
- , Rachel A. Freedman
- , Karen Burns White
- , Christine Gunn
- , Beverly Moy
- , Jennifer S. Haas
- , Caylin Marotta
- , Amy J Wint
- , Karen N. Freund
- , Amy M. LeClair
- , Susan K. Parsons
- , Feng Qing Wang
- , Serena Rajabiun
- & Stephenie C. Lemon
Contributions
SCL, AML, EC, DA, HC, JSH, KNH and TAB contributed to student conception and design. AML, EC, DA, MF, FQW, JR and EO contributed to the collection and assembly of data. SCL, AML, EC, DA, MF, HC, CLT and TAB contributed to data analysis. SCL, AML, EC, DA, HC, JSH, CC, KNF and TAB contributed to manuscript writing. All authors contributed to data interpretation and approved the manuscript.
Corresponding author
Correspondence to Stephenie C. Lemon .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the Integrated Network for Subject Protection In Research (INSPIR) at Boston University School of Medicine (docket H-37314). All participants in qualitative methods went through an informed assent process before agreeing to participate in this research. Patients received patient navigation services as part of standard of care and informed consent was not required, per INSPIR approval. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lemon, S.C., LeClair, A.M., Christenson, E. et al. Implementation of social needs screening for minoritized patients newly diagnosed with breast cancer: a mixed methods evaluation in a pragmatic patient navigation trial. BMC Health Serv Res 24 , 783 (2024). https://doi.org/10.1186/s12913-024-11213-7
Download citation
Received : 27 September 2023
Accepted : 17 June 2024
Published : 09 July 2024
DOI : https://doi.org/10.1186/s12913-024-11213-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Patient navigation
- Breast cancer
- Social needs
- Implementation
- Mixed methods
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
- Search Menu
- Sign in through your institution
- Advance Articles
- Supplements
- Special Series
- Breast Cancer
- Cancer Diagnostics and Molecular Pathology
- Community Outreach
- Endocrinology
- Gastrointestinal Cancer
- Genitourinary Cancer
- Geriatric Oncology
- Global Health and Cancer
- Gynecologic Oncology
- Head and Neck Cancers
- Health Outcomes and Economics of Cancer Care
- Hematologic Malignancies
- Hepatobiliary
- Immuno-Oncology
- Lung Cancer
- Medical Ethics
- Melanoma and Cutaneous Malignancies
- Neuro-Oncology
- New Drug Development and Clinical Pharmacology
- Pediatric Oncology
- Radiation Oncology
- Browse content in Regulatory Issues
- Regulatory Issues: FDA
- Regulatory Issues: EMA
- Symptom Management and Supportive Care
- Author Guidelines
- Submission Site
- Why Publish With Us?
- Open Access Options
- Self-Archiving Policy
- Advertising & Corporate Services
- Reprints, ePrints, Supplements
- About The Oncologist
- About the Society for Translational Oncology
- Editorial Board
- Discussions with Don S. Dizon
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, resulting findings, author contributions, conflicts of interest, data availability.
- < Previous
High-value breast cancer care within resource limitations
- Article contents
- Figures & tables
- Supplementary Data
Didier Verhoeven, Sabine Siesling, Claudia Allemani, Pankaj Gupta Roy, Luzia Travado, Nirmala Bhoo-Pathy, Clifford Rhayns, Hans Junkermann, Seigo Nakamura, Nwamaka Lasebikan, Forrest Lee Tucker, High-value breast cancer care within resource limitations, The Oncologist , Volume 29, Issue 7, July 2024, Pages e899–e909, https://doi.org/10.1093/oncolo/oyae080
- Permissions Icon Permissions
Breast cancer care is a costly global health issue where effective management depends on early detection and treatment. A breast cancer diagnosis can result in financial catastrophe especially in low- and middle-income countries (LMIC). Large inequities in breast cancer care are observed and represent a global challenge to caregivers and patients. Strategies to improve early diagnosis include awareness and clinical breast examination in LMIC, and screening in high-income countries (HIC). The use of clinical guidelines for the management of breast cancer is needed. Adapted guidelines from HIC can address disparities in populations with limited resources. Locally developed strategies still provide effective guidance in improving survival. Integrated practice units (IPU) with timely multidisciplinary breast care conferences and patient navigators are required to achieve high-value, personalized breast cancer management in HIC as well as LMIC. Breast cancer patient care should include a quality of life evaluation using ideally patient-reported outcomes (PROM) and experience measurements (PREM). Evaluation of breast cancer outcomes must include the financial cost of delivered care. The resulting value perspective should guide resource allocation and program priorities. The value of care must be improved by translating the findings of social and economic research into practice and resolving systemic inequity in clinical breast cancer research. Cancer survivorship programs must be put in place everywhere. The treatment of patients with metastatic breast cancer must require more attention in the future, especially in LMIC.
A global, coordinated alliance among breast cancer clinicians, advocates, management specialists, and others can optimize the quality of breast cancer care and value across diverse socio-economic, political, and geographic environments. To integrate a value quotient into breast cancer care, we must shift our focus from volumes to values and emphasize patient-centered outcomes as important metrics. Improved clinical outcomes with lower costs may require a transformation of health delivery systems including better organized public health systems with universal health coverage, and the development of public-private partnerships where appropriate.
Undesirable variations in early diagnosis, treatment, and quality management of breast cancer lead to suboptimal outcomes worldwide. Survival varies hugely worldwide. 1 Breast cancer deaths disproportionately affect women, especially in LMIC. To achieve improved outcomes for all patients with breast cancer worldwide, engagement of all stakeholders and a coordinated approach of their efforts is needed. 2 Strategies for breast cancer care delivery will be defined. These must be adapted to all socio-economic environments worldwide, reflecting their needs and capabilities, including human and infrastructural resources. Breast cancer can be considered as a global sentinel disease. The principles and structural aspects of an optimal care delivery model, having similar attributes applicable for many other chronic diseases, eg, other malignancies such as colorectal cancer and cervical cancer, diabetes, hypertension, and coronary artery disease, will be discussed. In early 2020, “Breast cancer: Global Quality Care” was published by Oxford University Press. 3 The reviewers confirmed the absence, but also the need for a more global and comprehensive vision of the disease. The need for a global strategy was supported by the first report of the Lancet Commission on breast cancer, 4 as well as by the WHO Global Breast Cancer Initiative (GBCI). The purpose of the GBCI is to reduce breast cancer mortality by 2.5% per year. Accordingly, the GBCI focuses on 3 main aspects of breast health: health promotion, timely diagnosis, and comprehensive treatment, including survivorship. Furthermore, we will try to identify the challenges and needs of breast cancer care delivery worldwide.
During the last 5 years, a faculty of 150 experts from 5 continents and 30 different countries was established. We communicated during expert meetings, mail, virtual conferences, webinars, and phone conversations to define a collaborative vision on quality breast cancer care. We collected the insights and perspectives of these leading experts including physicians, epidemiologists, economists, pharmacists, psychologists, nurses, patient advocates, journalists, and academics. This project focuses on the melding of high-quality value-based breast cancer care influenced by local economics and resources. The project is continuously under review by the faculty.
The relevant literature was screened from PubMed (MEDLINE), by using the following search terms: “breast cancer,” “value based health care or quality care or quality management or high value care,” and “resource limitations or low-middle income country or low-income country or resource limited country.” The presence of a limited literature was confirmed. Only 17 publications were found, from whom 5 were considered relevant. These findings justify this expert opinion review.
From the international faculty, 11 experts with expertise in value-based health care were selected coming from high-income countries (HIC; D.V., S.S., C.A., L.T., C.R., H.J., S.N., and L.T.) and LMIC (P.R., N.B.P., and N.L.) to discuss more in detail high-value breast cancer care, trying to define recommendations for better care. The different subjects of this review were divided among the experts:
Worldwide trends in population-based breast cancer survival were provided by the CONCORD programme (C.A.).
A review-based analysis was made about the advantages and outcomes of screening, breast awareness, and clinical breast examination (CBE; S.S., H.J., and N.L).
Health care spending data of some LMIC (Nigeria, Malaysia, and India) were provided (N.L., N.B.P., and P.R.) in addition to the World Bank and OECD data (L.T.).
Data about breast cancer research in some LMIC were described (N.B.P.).
The burden of breast cancer: a global problem
Surveillance of incidence and survival of breast cancer is key to understand the global picture of this disease, to study disparities, and to improve clinical and patient-reported performance.
Breast cancer is now the most common noncutaneous cancer in 140 of 184 countries in the world. 5 The 5-year estimated world prevalence was 6.2 million persons in 2012. 6 North America and Europe account for 9.4% and 25.3%, respectively, of the global number of deaths, although mortality rates are decreasing on both continents. The global proportion of deaths is 41% in Asia, 8.3% in Central and South America, 6% in North Africa and Western Asia, and 9% in sub-Saharan Africa. Breast cancer mortality rates are increasing in these world regions. 7
Population-based survival for patients diagnosed with breast cancer is a key measure of the overall effectiveness of health systems in detecting and managing the disease. This indicator summarizes the efficiency of early diagnosis, screening, treatment, and the availability of resources and local organization of breast cancer care. Global surveillance of breast cancer survival is meaningful only if we routinely monitor trends to assess whether improvement is being achieved. The CONCORD programme for the global surveillance of cancer survival is an example of such a worldwide initiative. 1 CONCORD-3 highlighted huge differences in age-standardized 5-year net survival worldwide for women diagnosed during 2010-2014, from 66% in India to 90% or more in the United States and Australia.
Despite being a high-income country, in the United States, age-standardized 5-year net survival was more than 10% lower for Black women than for White women, and this difference persisted over time (76.9% for Black women vs 89.1% for White women, in 2001-2003; 78.4% vs 89.6%, in 2004-2009). 8
Ethnic and socio-economic inequity is also suggested by the differing Black/White breast cancer mortality rates in the United States, ranging from nearly no difference in Massachusetts and Connecticut (northern states) to more than 1.5 in Louisiana and Mississippi (southern states). 9
Early diagnosis: awareness versus CBE versus screening
Survival from breast cancer is stage-dependent and significantly influenced by both early diagnosis and treatment of symptomatic disease, as well as mammography screening programs.
One of the key quality indicators in the assessment of breast cancer care worldwide is the stage at first diagnosis, which is strongly associated with the human development index (HDI). 10 The HDI adds the dimensions of life expectancy and education level to national per capita income as a means to stratify a nation’s resource environment ( Figure 1 , 11 ).

Relation between HDI and Stage I breast cancer Rene Aloisio da Costa Vieira et al. 11
While overviews 12 , 13 confirm the effectiveness of mammography screening and international guidelines 14 reflect the conviction of the large majority of experts that the usefulness of mammography screening has been proven beyond doubt, some questions are occasionally still raised about efficacy and effectiveness. 15 , 16 The real challenge is not to find an early disease but to avoid an advanced disease. The most reliable early indicator of the efficacy of a screening program is the reduction of the incidence of advanced-stage cancers in the population offered screening. This is a prerequisite for its effectiveness, leading to a decline in mortality eventually. Recent observational studies have shown the reduction of advanced disease in screening participants in the steady state of a long-term screening program in the Netherlands. 17 Irrespective of the classification used (TNM or NM), 18 screen-detected breast cancers were diagnosed less frequently at an advanced stage ( Figure 2A , 2B ). Also in Germany, after the introduction of mammography screening from 2005 to 2009, a fall of the incidence rate of advanced breast cancers was shown restricted to the target age group (50-69 years; Figure 2C , 2D ). 19 The failure to find such an effect in screening programs of established quality 20 must be attributed to methodological shortcomings. 21 In Germany, breast cancer mortality declined in the target age groups, while staying constant in younger or even further rising in older age groups. 19 The beneficial effect of screening has been maintained under conditions of nowadays Western World breast cancer awareness and advanced modern therapy.

(A, B) Trends in advanced breast cancer incidence in the screen-detected, interval, and non-screened cohorts in The Netherlands. The solid line indicates the screen-detected cancers assuming 10% overdiagnosis. The shaded area indicates the percentage assuming 0% overdiagnosis (lower limit) to 30% overdiagnosis (upper limit; de Munck et al 17 ). (C, D) Trends in breast cancer incidence in the screening age groups and stage in Germany. Y -axis: age-specific rates/100 000 women on a logarithmic scale. Dots: observed rates, lines rates by join-point regression, vertical dotted line: year implementation of screening (Katalinic et al 19 ).
Mammographic screening like any screening for asymptomatic breast cancer also has inherent inevitable negative effects, such as false positive results with cost and stress before a full assessment can exclude malignancy, leading to overdiagnosis, ie, breast cancers which would not have caused complaints, nor have been detected and treated during the remaining lifetime of the individual woman. Moreover, premalignant lesions as DCIS (ductal carcinoma in situ), which have a variable risk of becoming invasive, are detected frequently through the screening programs. The question of overtreatment of low-risk DCIS is currently addressed in several trials (LORIS, LORD, and COMET). 22 Quality control is of paramount importance to achieve and maintain a positive balance between positive and negative effects of screening programs. Personalized screening is now being tested with the aim of further improving this balance. 23
Though a recent review has stated that mammography screening would be cost-effective also in LMIC, 24 especially in the Upper-MIC, mammography screening is mostly not a relevant tool in LMIC due to its complexities, cost, and high infrastructural needs. In addition, a lack of funding might not support widespread national mammographic screening programs.
Clinical breast examination is often regarded as a better option for screening in resource-limited countries. In contrast to the high level evidence on the effectiveness of mammography screening, the evidence for the effectiveness of CBE is still scarce. An overview of meta-analyses of randomized studies on CBE 25 concluded in 2020: “There is no ‘direct’ evidence (from RCTs which compared CBE with no screening) that CBE is effective in terms of reducing breast cancer mortality.” The Canadian National Breast Screening Study (CNBSS) has been considered by some as indirect evidence of the equivalence of CBE with mammography as a screening method. 26 Recent evidence, however, documents the long-time suspected corruption of the randomization process in parts of this study invalidating its results. 27
Results on CBE screening from a randomized trial in Mumbai, 28 showing that clinical examination of asymptomatic women performed by well-trained personnel may have an effect on breast cancer mortality, should be viewed with caution. Significance was only found in a post hoc analysis of women older than 50 years without any effect in the younger population. The impact would also be restricted because in India, as in most LMIC, the peak of breast cancer mortality is below the age of 50 years. 29 Recent results from another randomized study of CBE screening in India did not find an effect on breast cancer mortality in any age group although as in the “Mumbai downstaging” was achieved. 30
In LMIC, the individual status of society regarding education and development of the health care system must be taken into account. Only if the health care system is sufficiently developed to offer adequate treatment for the population and the financial resources are available mammography screening may be an option. 31
For the majority of LMIC, however, the main priority is to advance diagnosis and treatment of symptomatic disease in order to avoid progression to advanced stages. Delays leading to stage progression have been shown to significantly impair survival. 32
Since the incidence is rising (see “The burden of breast cancer: a global problem” section), increasing the public awareness of the signs and symptoms of breast cancer is the first step in the implementation of an early detection program. Strategies incorporating breast cancer awareness and equipping health workers with skills to perform quality CBEs potentially play a role in the downstaging of cancers at diagnosis. 33
Not only financial limitations have to be overcome to reduce the load of advanced breast cancer. 34 Additional low health literacy, fear and cultural beliefs, high out-of-pocket treatment costs, lack of basic equipment, knowledge, training and skills of health professionals, shortage of specialist staff, difficult access to facilities by over-centralization, and poor communication have been identified in Zimbabwe 35 , 36 and in the Philippines. 37 Owing to such factors, 42% of women with suspicious finding on CBE actively refused further assessment in a randomized CBE screening study in the Philippines. 38 Such factors also have to be considered in certain groups and locations in HIC. Looking more in detail on-screen attendance reveals locales where this indicator is only 5%, even in the United States. Prioritizing resources to identify and screen these population subsets improves the identification of prevalent cancers at an earlier stage (L. Tucker, Virginia, United States, Personal written communication May 16, 2022). 39 , 40
Value-based breast care experience: “more than survival”
Having a diagnosis of breast cancer and undergoing treatment is an emotionally distressing event in a person’s life: it may produce psychological suffering which may impact the patient’s quality of life and survival. 41 However, distress can be easily screened by the Distress Thermometer, 42 which is a visual analog scale (from 0 to 10) to rate the level of distress a patient has felt in the past week. It allows for easy screening of psychosocial needs by including a checklist of physical, emotional, social, practical, and spiritual problems. In a landmark study on the prevalence of distress in patients with cancer, 32% of patients with early breast cancer reported high levels of emotional distress, 43 which increased by 60% in a metastatic phase and continued to increase with the progression of the disease toward the end-of-life. 44 Severe distress and depression if untreated lead to diminished quality of life, higher clinical complications, shorter survival, and increased health care costs. 45 Therefore, it is important to screen for the patient’s distress and psychosocial needs early in the treatment pathway to provide adequate psychological support and optimize patients’ well-being and clinical outcomes. This is now considered a quality standard of care for the treatment of patients with breast cancer and a requirement for the European certification of breast units. 46
Distress management should follow clinical guidelines. A 4-tiered model of professional psychological assessment and support has been recommended to address these needs to organize care in the clinic, which can be adjusted to each country’s available resources. All patients need effective information given through compassionate communication skills to reduce their anxiety related to their disease and treatment, in addition to general psychological/emotional support. This model recommends that this is the first basic level of emotional support which should be delivered by all direct health care professionals (eg, doctors and nurses). Good doctor/provider-patient communication is the essence of higher patient adaptation, higher patient compliance with treatment and care, higher patient satisfaction, and better patient clinical outcomes. 47 However, for patients with higher levels of distress, the model suggests the involvement of trained professionals in psychosocial oncology care, or mental health professionals, using evidence-based interventions to reduce patients’ emotional suffering. 48
The recently published essential requirements for quality cancer care of patients with breast cancer 46 reinforces the importance of psychologists in the multidisciplinary team in HIC, as well as LMIC, working in an integrated way to assure the patient the best outcomes and quality of life. Patients also require ongoing support in recovering from long and medium-term side-effects of treatments. Cognitive changes, sleep disturbances, fatigue, hot flashes, mood swings, depression, fear of recurrence, self-image alterations, loss of libido and sexual alterations interfere with normal return to work, and resumption of life, family, and social roles. Survivorship care is a much-neglected area and we need to develop programs for better support our patients in resuming a normal life. The European Guide on Quality Improvement in Comprehensive Cancer Control does recommend a survivorship care plan that enhances patient’s self-management and quality of life. 49
Value Quotient Breast Care may be defined as improved patient-centered outcomes (survival and well-being), following the identification and treatment of a benign or malignant breast abnormality against the costs of full-cycle clinical management. Patient management expenses are directly related to the extent of clinical interventions, which are proportionate to the stage at diagnosis. 50 , 51
What is often missed when analyzing the expenses following a cancer diagnosis is that a lack of well-being in patients may lead to higher direct and indirect costs. In many resource-limited settings, the measure of success in cancer care and control is heavily focused on survival. “How well” patients live following a cancer diagnosis, as well as “how long” they live should be acknowledged as important. Patient-centered outcomes such as quality of life and return to work offer important insights into the value of breast cancer care. 52
Health executives, policymakers, clinicians, and patient advocates must collaborate to design and implement comprehensive breast care services, encompassing the full cycle of breast health from the asymptomatic individual presenting for screening through diagnosis, treatment, supportive care, survivorship phase, and end of life. These initiatives are necessary to address challenges and opportunities to improve breast care value across diverse geopolitical and socio-economic environments.
Common to all scenarios is the desirability of a functioning breast integrated practice unit (IPU) with a focus on performance data collection and informed decision-making for all aspects of service and patient outcomes. The establishment of breast IPUs with a regular, (at least weekly) multidisciplinary breast cancer planning conference must be considered a major public health achievement. 53 This team evaluates patient-specific disease attributes to recommend the best, personalized, and cost-effective diagnostic and treatment options. Irrespective of whether the clinical environment is HIC or LMIC, the IPU must adopt a culture of performance measurement with the adoption of clinically and financially relevant and actionable metrics. Clinical and financial outcome measures must be regularly and critically reviewed by clinicians and managers empowered to improve performance.
Patient navigators serve an essential role in the improvement of the patient experience and outcomes as patient advocates in the multidisciplinary team. 54 Navigators are well-positioned to coordinate the care of individual patients. When provided with clinical practice protocols, navigators can facilitate the diagnostic workup and reduce or eliminate over- and under diagnosis. Similarly, by coordinating care across the care continuum, including medical genetics, surgery, reconstruction, medical and radiation oncology, and survivorship, navigators can assist in the reduction of both over-and under treatment.
Based on the Value-Based Health Care strategic framework defined by Porter 55 and Teisberg, 56 the following key steps are proposed for implementation ( Figure 3 ):

Value-Based Health Care framework, from Teisberg 56 .
The clinical and psychosocial needs of patients with breast cancer must be understood.
Coordination of care in IPUs to improve performance from community screening to survivorship and end-of-life care:
◦ in breast care, accomplished with comprehensive, interdisciplinary centers;
◦ multidisciplinary discussions must be mandatory with expertise of different disciplines.
Integrated learning teams must be formed:
◦ in breast care, this includes marketing, information technology, administration, social services, and rehabilitation;
Managing costs: how do we reduce the cost of provided care?
◦ in many LMIC, costs are often perceived as too high, influencing the decision to seek care. Many patients are paying for care out of pocket, with the added costs becoming unbearable;
◦ begin with stage shifting through clinical and mammographic screening (reduced reliance on tertiary care);
◦ imaging-guided biopsy replacing surgical (especially in LMIC).
Expanding better organized public health systems/extending the influence of clinical breast centers:
◦ providing guidance to breast cancer advocacy groups, payers, and policymakers;
◦ social media;
◦ discussing the opportunities and pitfalls of public-private partnerships;
◦ integration of radiology, pathology, surgery, and oncology clinical services;
◦ extend local hospital or clinic services to expand community health initiatives (merging of local breast units or centers into regional health systems with the ability to serve large geographic regions and leverage resources).
Limitations of global breast care delivery
Health care spending per capita by source of funding varies considerably among HIC, 57 LMIC, and LIC as demonstrated in Figure 4 . The understanding of the cost in LMIC is limited but critical to guide effective delivery strategies. 58 There has been nevertheless substantial growth in the number of breast cancer economic evaluations in LMICs in the past decade. The per capita health care spending in 2019 was reported by the World bank database ( www.dataworldbank.org ) to be 34 in LIC, 96 in LMIC, and 5635 in HIC. 59 In Malaysia, health financing is largely subsidized by public funding approximating 51% of total health spending. An important issue is out-of-pocket expense and medical care, which is not reimbursed by insurance or government payments. In 2020, this out-of-pocket spending soared to 43% of total health spending in the nation. Nigeria’s health spending per capita remains even lower but with an out-of-pocket expenditure on health estimated at three-quarters of the nation’s health expenditure in 2018. A large percentage of the population is unable to afford—and have limited access to—cancer treatment services. 60 In LMIC, and even in some HIC, a breast cancer diagnosis can be a financial catastrophe with important bill problems for the patient and family. Medical bill problems are defined as unexpected insurance denials, co-pays, deductibles, or out-of-pocket expenses. 61 The pooled rate of financial toxicity for patients with breast cancer was 78% in low- and middle-income countries and 35% in HIC. 62

Health care spending per capita US dollar (World Bank Open data, 2019, retrieved January 30, 2022).
Societal health care expenditures, of which a considerable part is due to breast cancer care, are rising to levels that may not be sustainable in the future. Cancer causes high costs both within and outside the health care system, in part due to the rising cost of cancer drugs. Economic evaluations of new and existing therapies can be used to inform budget allocations in a way that maximizes health outcomes and broader value to the patient. It is increasingly recognized that personalized care, defined as a better selection of those patients getting most advantage of treatment, can offer more value for patients and at the same time provide value for money. It is timely that current clinical practice guidelines are revisited toward this personalized approach, acknowledging the patient’s voice, as well as the cost to society of therapy. The best example is the correct identification of the receptor and Her2 status. These data are many times missing in LMIC. Another example is the recognition of the health assessments to identify the appropriateness of therapy and the calculation of the survival gain of different treatments. The possibility of de-escalation of 12- to 6-month adjuvant trastuzumab can be considered as an example of decision-making in a resource-limited setting, but hampered by the difficulty in changing international guidelines. A 12-month regimen of trastuzumab had an additional annual cost of US$6 million in Peru, being too expensive considering the limited budget. 63 Hypofractionation of radiation therapy, from 25 to 5 fractions is another example of introducing cost-efficiency. 64 The use of clinical benefit scales of the American Society of Clinical Oncology (ASCO) and the European Society of Medical Oncology (ESMO) are considered valuable tools but have not gained enough acceptance. 65 , 66
Breast cancer care is hampered by numerous local circumstances, including variations in availability and access to resources, administrative efficiency, and organization of the care process. The differences in access are related to local affordability and timeliness of care. Timeliness is defined by wait time for appointments and time to obtain information and reports. Affordability means the ability to pay for the care, such as having an insurance to cover the expenses with minimal out-of-pocket cost. Significant disparities are reported by the OECD in the health care performance even among different HIC ( https://worldpopulationreview.com/country-rankings/cancer-survival-by-country ). National strategies and policies should ideally be inspired by the best available models to reduce the financial burden of a breast cancer diagnosis. Tailoring clinical practice guidelines to the local context results in a valuable, resource-efficient tool that can be used by health professionals and patients to focus on ethnic differences and the assumption of different biologic behavior between different races and nations. 67 , 68 An example is the collaboration between ESMO and the Japanese Society of Medical Oncology started in 2016, becoming an active partnership between the oncology societies of China, India, Indonesia, South Korea, Malaysia, Philippines, Singapore, Taiwan, and Thailand integrating ethnic, scientific, socioeconomic, and local practice characteristics. 69
Evaluation of breast cancer care must also give special attention to the reduction of “avoidable deaths,” defined in this context as deaths from all causes that are considered to have been due to medical or laboratory errors. Important differences remain among OECD members states not only in the proportion of avoidable deaths (mean of 199 deaths per 100 000 population in OECD countries, ranging from 139 in Australia, 191 in Chili, 216 in Turkey, 265 in United States to 366 in Mexico) but also in each country’s success in reducing these deaths. 70 A poor performance suggests a worse access to primary care, prevention, and chronic disease management.
Clinical breast cancer research in vulnerable populations
A look at the world map of clinical breast cancer studies showed that in 2022, only 246 clinical trials were registered in Africa, 388 studies in South America, 161 in India, and 269 studies in Southeast Asia, compared to 3135 studies in Europe. 71 In contrast, there appears to be a rapid increase in China with 1635 clinical trials registered in 2022. Enrollment in clinical trials must reflect the demographic diversity of people of the health condition under study. Barriers to the participation of marginalized communities must be removed. 72 In addition, more attention must go to include members of ethnic minorities, people with disabilities and geriatric populations. Initiatives from all parties in clinical and translational research are needed to translate biomedical discoveries into health equity for all. The right questions must be asked of the representative patient populations to receive the right answers. More attention must go to real-world evidence with attention to benefit and risk derived from the analysis of real-world data. In their recent draft guidance, the US Food and Drug Administration (FDA) discusses the use of real-world data in support of decision-making about the safety and efficiency of new drugs. 73 Electronic health records, medical claims data, and patient registries must all be evaluated. Breast cancer management strategies in the LMICs must not adopt but rather adapt Western knowledge as most of the current knowledge on breast cancer has been generated in Western populations. As an example, socio-economic profiles, life style, culture, and genetic background of Asian and Western women are substantially different from each other. 74
An illustrative example is the research on artificial intelligence with thermal images of early breast cancer developed in India. 75 The proportion of dense breast is almost twice in Asians and Africans compared with Caucasians. Therefore, classical mammography screening might be less adequate for Asians and Africans.
Indirect cost rate contributes importantly to research inequity in global health research. Additional funding for this cost can provide critical support for infrastructure and facility operations fueling the capacity to conduct more research in LMIC. Discussions with international research partners on how to use investments more adequately could strengthen global research. 76 In addition, more studies must focus on survivorship and patient-centered outcomes in these populations.
Breast cancer: a global problem
Breast cancer is a major global health problem with increasing incidence, especially in LMIC. Monitoring worldwide survival trends is a key to formulating strategies for global breast cancer control, as shown by the CONCORD programme.
In HIC, mammographic screening of the asymptomatic population has been effective in shifting diagnosis to an earlier stage. The critical issue of obtaining less advanced-stage breast cancer after the introduction of a screening program must be established. Strategies to improve cancer detection in LMIC should emphasize the development of national breast cancer networks to coordinate care and to promote clinical early detection. Efforts to increase early detection strategies should accompany those to increase access to treatment. As such, costly community mammographic screening detection initiatives may be a priority only when implemented following the deployment of clinical evaluation protocols for symptomatic and asymptomatic individuals with available access to quality treatment. Treatment facilities must be strengthened to accommodate the accompanying volume as most treatment facilities in LMIC have limited human and infrastructural capacity, with fragile health systems that can be easily overwhelmed. In LMIC, cultural, economic, and logistic barriers may render mammographic screening an inefficient method for the early detection of breast cancer. Findings about CBE from a large, randomized clinical trial in Mumbai are viewed with optimism by the global cancer community. The situation in each country must be analyzed individually before an action plan can be implemented. All breast cancer care activities must be developed in a coordinated pattern to achieve the desired results avoiding low-value or harmful practices. 77 The WHO’s GBCI develops resource-stratified guidelines for the implementation of early detection and therapy of breast cancer programs.
Strategies to improve high-value breast cancer care have to be defined in HIC, but even more in LMIC. These strategies for care delivery must be scalable and appropriate for diverse socio-economic environments worldwide, reflecting the different needs of LMIC and HIC. The principles and structural aspects of an optimal care delivery model include specialized clinical leadership, regularly updated clinical guidelines, multidisciplinary coordination of care, and rigorous measurement of clinical and value quotient outcomes including PROMs and PREMs. The value should be defined around the patient with breast cancer, in a well-functioning health care system. The creation of value could even determine the rewards available to care providers. The multidisciplinary breast cancer conference is considered to be “the jewel in the crown” of the IPU, coordinating multiple specialties and functions around patients with breast cancer. Their task is to define personalized treatment opportunities for shared decision-making with the patient discussing the best opportunities taking into account available resources.
Financial toxicities among patients with breast cancer are substantially higher than among other health conditions. While data on spending for breast cancer care are largely unavailable in the LMICs, it is conceivable that lack of funding for health care and rising OOP spending in these countries will have a detrimental impact on cancer care delivery and the financial well-being of households affected by breast cancer. Innovations such as precision medicine may help reduce over- and under treatment but must be evaluated from a rigorous value perspective. The costs associated with specific diagnostic studies and treatments hamper access: drugs do not benefit those who cannot afford them. Transparent, fair, and evidence-based decision-making with a value quotient perspective must guide the allocation of our limited resources to achieve high-quality care. Entry agreements with pharmaceutical companies may be used to manage risks when a therapy lacks supportive clinical evidence. The value perspective must also be used to support shared decision-making by integrating patient-reported outcomes, clinical evidence, and broader societal considerations. Equity in breast cancer care must be ensured for all patients with breast cancer. Justice can only be provided by fixing the system to offer equal access in LMIC but also in HIC to both tools and opportunities. 78 There is an urgent need for more resources to aid early detection and provide financial protection from the cost of a cancer diagnosis. Utilizing existing community platforms such as HIV awareness programs could also improve breast cancer awareness and CBE practices.
Periodical comparison of key quality indicators
Identification and reporting of some key quality indicators are a minimal requirement in HIC and LMIC. Most of them were suggested by the Breast Health Global Initiative (quality indicators 2, 4 and 5):
real-world population-based survival estimates, as shown by the CONCORD programme;
stage distribution at first diagnosis, with a minimum level of 60% of stage I or II (BHGI data);
distribution of ER, PgR, and HER2 status examination;
diagnostic interval of maximally 60 days between first observation and the start of therapy (BHGI data); and
80% of patients who accomplished the proposed treatment (BHGI data).
There is a clear need for research in vulnerable populations adapted to the local environments, taken into account ethnic differences, local resources, and local organization of breast cancer care. Apart from intervention studies, diagnostic and prognostic studies are also local-specific and have to be validated in LMICs before implementation in their routine clinical practice.
Conception/design: Didier Verhoeven, F. Lee Tucker. Manuscript writing and final approval of manuscript: All authors.
The authors declared no conflicts of interest.
No new data were generated or analyzed in support of this research.
Allemani C , Matsuda T , Di Carlo V , et al. ; CONCORD Working Group . Global surveillance of trends in cancer survival 2000-2014 (CONCORD-3): analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries . Lancet . 2018 ; 391 ( 10125 ): 1023 - 1075 . https://doi.org/10.1016/S0140-6736(17)33326-3
Google Scholar
Verhoeven D , Allemani C , Kaufman C , et al. . New frontiers for fairer breast cancer care in a globalized world . Eur J Breast Health . 2021 ; 17 ( 2 ): 86 - 94 . https://doi.org/10.4274/ejbh.galenos.2021.2021-1-1
Verhoeven D , Kaufman C , Mansel R , Siesling S. Breast Cancer: Global Quality Care . OUP ; 2020 .
Google Preview
Coles C , Anderson B , Cameron D , et al. . The lancet breast cancer commission: tackling a global health, gender and equity challenge . Lancet . 2022 ; 399 ( 10330 ): 1101 - 1103 .
Bray F , Colombet M , Mery L , et al. eds. Cancer incidence in five continents . Vol XI. IARC Scientific Publication No. 166.; Lyon: International Agency for Research on Cancer. Available from: https://publications.iarc.fr.597.Licence : CC BY-NC-ND 3.0 IGO.
Ferlay J , Ervik M , Lam F , et al. . Global Cancer Observatory: Cancer Today . Lyon, France : International Agency for Research on Cancer. Available from : https://gco.iarc.who.int/today , accessed 2016 . http://gco.iarc.fr/today
De Santis C , Bray F , Ferlay J , et al. . International variation in female breast cancer incidence and mortality rates . Cancer Epidemiol Biomarkers Prev . 2015 ; 24 ( 10 ): 1495 - 1506 .
Miller J , Smith L , Ryerson AB , et al. . Disparities in breast cancer survival in the United States (2001-2009): findings from the CONCORD-2 study . Cancer . 2017 ; 123 ( Suppl. 24 ): 5100 - 5118 .
De Santis C , Ma J , Sauer A , et al. . Breast cancer statistics, 2017, racial disparities in mortality by state . CA Cancer J Clin . 2017 ; 67 ( 6 ): 439 - 448 .
Max R. Human Development Index . 2014 . Accessed April 9, 2022. https://OurWorldInData.org/human-development-index
Rene Aloisio da Costa V , Biller G , Uemura G , et al. . Breast cancer screening in developing countries . Clinics (Sao Paulo) . 2017 ; 72 ( 4 ): 244 - 253 .
Health Council of the Netherlands . Population Screening for Breast Cancer: Expectations and Developments . Health Council of the Netherlands ; 2014 . Publication no. 2014/01E.
Independent UK Panel on Breast Cancer Screening . The benefits and harms of breast cancer screening: an independent review . Lancet . 2012 ; 380 ( 9855 ): 1778 - 1786 .
European Commission Initiative on Breast Cancer . https://healthcare-quality.jrc.ec.europa.eu/ecibc/european-breast-cancer-guidelines
de Glas N , de Craen A , Bastiaannet E , et al. . Effect of implementation of the mass breast cancer screening programme in older women in the Netherlands: population based study . BMJ. 2014 ; 349 : g5410 .
Autier P , Boniol M , Middleton R , et al. . Advanced breast cancer incidence following population-based mammographic screening . Ann Oncol . 2011 ; 22 ( 8 ): 1726 - 1735 . https://doi.org/10.1093/annonc/mdq633
de Munck L , Siesling S , Fracheboud J , et al. . Impact of mammographic screening and advanced cancer definition on the percentage of advanced-stage cancers in a steady-state breast screening programme in the Netherlands . Br J Cancer . 2020 ; 123 ( 7 ): 1191 - 1197 .
Brierley JD , Gospodarowicz M , Wittekind C , eds. TNM Classification of Malignant Tumours . 8th ed. UICC ; 2017 .
Katalinic A , Eisemann N , Kraywinkel K , Noftz MR , Hübner J. Breast cancer incidence and mortality before and after implementation of the German mammography screening program . Int J Cancer . 2020 ; 147 ( 3 ): 709 - 718 . https://doi.org/10.1002/ijc.32767
Waldmann A , Hübner J , Katalinic A. Trends over time in breast-cancer-specific mortality in Germany . Dtsch Arztebl Int . 2021 ; 118 ( 31-32 ): 538 - 539 . https://doi.org/10.3238/arztebl.m2021.0182
Ren W , Chen M , Qiao Y , Zhao F. Global guidelines for breast cancer screening: a systematic review . Breast . 2022 ; 64 : 85 - 99 . https://doi.org/10.1016/j.breast.2022.04.003
Co M , Ngan RKC , Mang OWK , et al. . Long-term survival outcomes of “low risk” ductal carcinoma in situ from a territory-wide cancer registry . Clin Oncol (R Coll Radiol) . 2021 ; 33 ( 1 ): 40 - 45 . https://doi.org/10.1016/j.clon.2020.07.007
Roux A , Cholerton R , Sicsic J , et al. . Study protocol comparing the ethical, psychological and socio-economic impact of personalised breast cancer screening to that of standard screening in the “My Personal Breast Screening” (MyPeBS) randomised clinical trial . BMC Cancer . 2022 ; 22 ( 1 ): 507 . https://doi.org/10.1186/s12885-022-09484-6
Icanervilia AV , van der Schans J , Cao Q , et al. . Economic evaluations of mammography to screen for breast cancer in low- and middle-income countries: a systematic review . J Glob Health . 2022 ; 12 : 04048 . https://doi.org/10.7189/jogh.12.04048
Ngan TT , Nguyen NTQ , Van Minh H , Donnelly M , O’Neill C. Effectiveness of clinical breast examination as a “stand-alone” screening modality: an overview of systematic reviews . BMC Cancer . 2020 ; 20 ( 1 ): 1070 . https://doi.org/10.1186/s12885-020-07521-w
Jean MS , Peter RE , Martin JY. The fundamental flaws of the CNBSS Trials: a scientific review . J . Breast Imaging . 2022 ; 4 ( 2 ): 108 - 119 .
Duffy SW. Problems with the Canadian national breast screening studies . J Breast Imaging . 2022 ; 4 ( 2 ): 120 - 121 .
Mittra I , Mishra GA , Dikshit RP , et al. . Effect of screening by clinical breast examination on breast cancer incidence and mortality after 20 years: prospective, cluster randomised controlled trial in Mumbai . BMJ . 2021 ; 372 : n256 . https://doi.org/10.1136/bmj.n256
Malvia S , Bagadi SA , Dubey US , Saxena S. Epidemiology of breast cancer in Indian women . Asia Pac J Clin Oncol . 2017 ; 13 ( 4 ): 289 - 295 . https://doi.org/10.1111/ajco.12661
Ramadas K , Basu P , Mathew BS , et al. . Effectiveness of triennial screening with clinical breast examination: 14-years follow-up outcomes of randomized clinical trial in Trivandrum, India . Cancer . 2023 ; 129 ( 2 ): 272 - 282 . https://doi.org/10.1002/cncr.34526
Ozkan Gurdal S , Ozaydın AN , Aribal E , et al. . Long-term population-based screening compared to National Breast Cancer Registry Data: effectiveness of screening in an emerging country . Diagn Interv Radiol . 2021 ; 27 ( 2 ): 157 - 163 . https://doi.org/10.5152/dir.2021.20486
Richards MA , Westcombe AM , Love SB , Littlejohns P , Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review . Lancet . 1999 ; 353 ( 9159 ): 1119 - 1126 . https://doi.org/10.1016/s0140-6736(99)02143-1
Ngwa W , Addai BW , Adewole I , et al. . Cancer in sub-Saharan Africa: a Lancet Oncology Commission . Lancet Oncol . 2022 ; 23 ( 6 ): e251 - e312 . https://doi.org/10.1016/S1470-2045(21)00720-8
Foerster M , McKenzie F , Zietsman A , et al. . Dissecting the journey to breast cancer diagnosis in sub-Saharan Africa: Findings from the multicountry ABC-DO cohort study . Int J Cancer . 2021 ; 148 ( 2 ): 340 - 351 . https://doi.org/10.1002/ijc.33209
Elmore S , Mushonga M , Subramaniam Iyer H , et al. . Breast cancer in Zimbabwe: patterns of care and correlates of adherence in a national refferal hospital radiotherapy center cohort from 2014 to 2018 . Cancer Med . 2021 ; 10 ( 11 ): 3489 - 3498 .
Magara M , Mungazi S , Gonde P , et al. . Factors leading to the late diagnosis and poor outcomes of breast cancer in Matabeleland South and the Bulawayo Metropolitan Provinces in Zimbabwe . PloS One. 2023 ; 18 ( 11 ); e0292169 .
Pisani P , Parkin DM , Ngelangel C , et al. . Outcome of screening by clinical examination of the breast in a trial in the Philippines . Int J Cancer . 2006 ; 118 ( 1 ): 149 - 154 . https://doi.org/10.1002/ijc.21343
Ho F , Arevalo M , de Claro P , et al. . Breast and cervical cancer screening in the Philippines: challenges and steps forward . Prev Med Rep . 2022 ; 29 101936 . https://doi.org/10.1016/j.pmedr.2022.101936 .
Tucker FL. Imaging-assisted large-format breast pathology: program rationale and development in a nonprofit health system in the United States . Int J Breast Cancer. 2012 ; 2012 : 171792 . https://doi.org/10.1155/2012/171792
Peralta EA , Tucker FL. Pre-operative magnetic resonance imaging and large-format breast pathology: closing the loop . J Clin Oncol . 2014 ; 32 ( 25 ): 2817 - 2818 . https://doi.org/10.1200/JCO.2014.56.3361
Stagl JM , Lechner SC , Carver CS , et al. . A randomized controlled trial of cognitive-behavioral stress management in breast cancer: survival and recurrence at 11-year follow-up . Breast Cancer Res Treat . 2015 ; 154 ( 2 ): 319 - 328 . https://doi.org/10.1007/s10549-015-3626-6
Riba MB , Donovan KA , Ahmed K , et al. NCCN Clinical Practice Guidelines in Oncology: Distress Management . Version 1. 2022 .
Zabora J , BrintzenhofeSzoc K , Curbow B , Hooker C , Piantadosi S. The prevalence of psychological distress by cancer site . Psychooncology . 2001 ; 10 ( 1 ): 19 - 28 . https://doi.org/10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6
Mosher CE , DuHamel KN. An examination of distress, sleep, and fatigue in metastatic breast cancer patients . Psychooncology . 2012 ; 21 ( 1 ): 100 - 107 . https://doi.org/10.1002/pon.1873
Travado L , Reis J , Antoni M. Prevalence of psychological distress, anxiety and depression in metastatic breast cancer patients. meeting abstract: world cancer congress . J Glob Oncol. 2018 ; 4 ( Suppl 2 ): 224s .
Biganzoli L , Cardoso F , Beishon M , et al. . The EUSOMA requirements of a specialist breast centre . Breast . 2020 ; 51 : 65 - 84 . https://doi.org/10.1016/j.breast.2020.02.003
NICE . Improving Supportive and Palliative Care for Adults with Cancer: The Manual. Guidance on Cancer Services . National Institute of Clinical Excellence, NHS ; 2004 .
Epstein RM , Street RL , Jr . Patient-Centered in Communication in Cancer Care: Promoting Healing and Reducing Suffering . National Cancer Institute, NIH ; 2007 . Publication No. 07-6225 .
Albreht T , Borrás-Andrés JM , Dalmas M , et al. Survivorship and rehabilitation: policy recommendations for quality improvement in cancer survivorship and rehabilitation in EU Member States . In: Albreht T , Kiasuwa R , Van den Bulcke M , eds. European Guide on Quality Improvement in Comprehensive Cancer Control . National Institute of Public Health; Scientific Institute of Public Health ; 2017 . https://cancercontrol.eu/archived/uploads/images/Guide/pdf/CanCon_Guide_FINAL_Web.pdf
Lemhouer H , Verhoeven D , Corluy V , et al. . The direct medical cost of breast cancer: a case study in one hospital . Belg J Med Oncol. 2020 ; 14 ( 1 ): 22 - 27 .
Blumen H , Fitch K , Polkus V. Comparison of treatment costs for breast cancer by tumor stage and type of service: a population based approach . Am Health Drug Benefits . 2016 ; 9 ( 1 ): 23 - 32 .
Sun Y , Shigaki CL , Armer J. Return to work among breast cancer survivors: a literature review . Support Care Cancer . 2017 ; 25 ( 3 ): 709 - 718 .
Verhoeven D , Allemani C , Kaufman C , et al. . Breast cancer: global quality care delivery with existing financial and personnel resources . ESMO Open . 2020 ; 4 ( Suppl 2 ): e000861 .
Dalton M , Holzman E , Erwin E , et al. . Patient navigation services for cancer care in low-and middle-income countries: a scoping review . PLoS One . 2019 ; 14 ( 10 ): e0223537 . https://doi.org/10.1371/journal.pone.0223537
Porter M. What is value in health care ? N Engl J Med . 2010 ; 363 ( 26 ): 2477 - 2472 .
Teisberg E , Wallace S , O’Hara S. Defining and implementing value-based health care: a strategic framework . Acad Med . 2020 ; 95 ( 5 ): 682 - 685 . https://doi.org/10.1097/ACM.0000000000003122
Tikkanen R , Melinda A. US Health Care From a Global Perspective, 2019. Higher Spending: Worse Outcomes? The Commonwealth Fund ; 2020 . Accessed April 9, 2022 . https://www.Commonwealthfund.org/publications/issue-briefs/2020/jan/us-health-care-global-perspective-2019 .
Erfani P , Bhangdia K , Stauber C , et al. . Economic evaluations of breast cancer in low- and middle-income countries: a scoping review . The Oncologist 2021 ; 26 ( 8 ): e1406 - e1417 .
World Bank Open Data , 2019 , retrieved Jan 30, 2022 . https://data.worldbank.org
Ndoh K , Aliko A , Yates R , et al. . Cancer control funding in nigeria: a case for universal health coverage . J Cancer Policy. 2022 ; 32 : 100335 .
Munira ZG , Roosa T , Shanoor S , et al. What is the Status of Women’s Health and Health Care in the US Compared to Ten Other Countries. Commonwealth Fund Issue Briefs . Single Payer New York ; 2018 .
Ehsan A , Wu C , Minasian A , et al. . Financial toxicity among patients with breast cancer worldwide. A systematic review and meta-analysis . JAMA Netw Open. 2023 ; 6 ( 2 ): e2255388 .
Becerra-Chauca N , Nieto-Gutierrez W , Taype-Rondan A. 6-Month or 12-month adjuvant trastuzumab regimen for HER2-positive breast cancer? Decision-making in a resource-limited setting . Lancet Oncol . 2022 ; 23 ( 3 ): e100 . https://doi.org/10.1016/S1470-2045(22)00060-2
Adrian MB , Joanne SH , Duncan AW , et al. , on behalf of the FAST-Forward Trial Management Group. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial . Lancet . 2020 ; 395 ( 10237 ): 1613 - 1626 .
Cherny N , Dafni U , Bogaerts J , et al. . ESMO-magnitude of clinical benefit scale version 1.1 . Ann Oncol . 2017 ; 28 ( 10 ): 2340 - 2366 .
Schnipper L , Davidson N , Wollins D , et al. . American society of clinical oncology statement: a conceptual framework to assess the value of cancer treatment options . J Clin Oncol . 2015 ; 33 ( 23 ): 2563 - 2577 .
Anderson BO , Ilbawi AM , Fidarova E , et al. . The global breast cancer initiative: a strategic collaboration to strengthen health care for non-communicable diseases . Lancet Oncol . 2021 ; 22 ( 5 ): 578 - 581 . https://doi.org/10.1016/S1470-2045(21)00071-1
Yip C , Darby R. Improving breast cancer outcomes in Asia Cancer Control 2017 ; 50 - 56
Park Y , Senkus-Konefka E , Im SA , et al. . Pan-Asian ADAPTED ESMO clinical practice guidelines for the management of patients with early breast cancer: a KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS . Ann Oncol . 2020 ; 31 ( 4 ): 451 - 469 .
Schneider E , Shah A , Doty M , et al. . Reflecting poorly: healthcare in the US compared to other high-income countries . Accessed April 9, 2022 . https://www.Commonwealthfund.org/publications/fund-reports/2021/aug/mirror-mirror-2021-reflecting-poorly
NIH . U.S. National Library of Medicine . Accessed April 9, 2022. www.clinicaltrials.gov
Boulware L , Corbie G , Aguilar-Gaxiola S , et al. . Combating structural inequities-diversity, equity, and inclusion in clinical and translational research . N Engl J Med . 2022 ; 386 ( 3 ): 201 - 203 .
Considerations for the Use of Real-World Data and Real-World Evidence to Support Regulatory Decision-Making for Drug and Biological Products. FDA guidance document for industry. August 2023. FDA-2021-D-1214 .
Nirmala BP , Cheng-Har Y , Hartman M , et al. . Breast cancer research in Asia: adopt or adapt Western knowledge ? Eur J Cancer . 2013 ; 49 ( 3 ): 703 - 709 .
Singh A , Bhat V , Sudhakar S , et al. . Multicentric study to evaluate the effectiveness of Thermalytix as compared with standard screening modalities in subjects who show possible symptoms of suspected breast cancer . BMJ Open. 2021 ; 11 ( 10 ): e052098 . https://doi.org/10.1136/bmjopen-2021-052098
Haberer J , Boum Y. Behind-the-scenes investment for equity in global health research . N Engl J Med . 2023 ; 388 ( 5 ): 387 - 390 .
Rubagumya F , Mitera G , Ka S , et al. . Choosing wisely Africa: ten low-value or harmful practices that should be avoided in cancer care . JCO Glob Oncol. 2020 ; 6 : 1192 - 1199 . https://doi.org/10.1200/GO.20.00255 .
What’s the difference between equity and equality ? Mental Floss, 2020 , Accessed April 9, 2022. www.mentalfloss.com/article/625404/equity-vs-equality-what-is-the-difference
| Month: | Total Views: |
|---|---|
| May 2024 | 368 |
| June 2024 | 324 |
| July 2024 | 69 |
Email alerts
Citing articles via.
- Advertising and Corporate Services
- Journals Career Network
Affiliations

- Online ISSN 1549-490X
- Print ISSN 1083-7159
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

IMAGES
VIDEO
COMMENTS
Breast cancer is the second leading cause of cancer mortality for US women, despite a steady overall decline in breast-cancer mortality rates over the past 20 years. 1 The average age-adjusted rate for the years 2016-2020 was 19.6 per 100 000, with an estimated 43 170 deaths in 2023. 1,2 The majority of cases occur between the ages of 55 and 74 ...
Three guidelines recommended screening every 1-2 years [ [8], [23], [32] ]. Some guidelines agreed that screening intervals should be determined based on age [ 18, 24 ]. ACS [ 23] recommended screening with MAM annually for women aged 40-54 years and every 1-2 years for women aged 55 years or older.
1. Introduction. In 2021, breast cancer has overtaken lung cancer to be the world's most commonly diagnosed cancer, accounting for the severe burden globally, especially among women [1].Screening for breast cancer is an effective measure to detect early-stage disease and improve the survival rate of cancer patients [[2], [3]].Population-based breast cancer screening programs have been ...
In a literature review, Leonard Fass (2008) and Safarpour Lima and colleagues (2019) found that cancer care is dependent on imaging through screening [ 11, 14 ]. Breast cancer can be detected early using imaging tools [ 6 ]. The sensitivity and specificity of various techniques, however, vary [ 15, 16 ]. Integrated imaging techniques, according ...
Systematic Review of Cancer Screening Literature for Updating American Cancer Society Breast Cancer Screening Guidelines Prepared for: American Cancer Society, Inc. 250 Williams Street Atlanta, GA 30303 Contract No. 13214 Prepared by: Duke Evidence Synthesis Group Durham, NC Investigators: Laura Havrilesky, M.D. Jennifer M Gierisch, Ph.D.
Abstract. The present study aims to systematically review the women's knowledge, attitude, and practice (KAP) of breast cancer (BC) screening methods to get enough information for policymakers to orient the screening strategies. All English KAP studies on BC screening methods in five databases up to January 2021 were included.
The purpose of this article is to review the evidence regarding breast cancer screening for average-risk women. The review primarily focuses on mammographic screening but also reviews clinical breast examinations, emerging screening technologies, and opportunities to build consensus. Wherever possible, the review relies on published systematic ...
Abstract. Purpose: To (a) conduct a thorough search of the literature for breast cancer screening studies utilizing mammography, ultrasound, or breast magnetic resonance imaging (MRI), and (b) critically appraise these studies to aid the nurse practitioner in choosing the most appropriate screening tool for their individual patients.
For average-risk women, most of the guidelines recommended mammographic screening for those aged 40-74 years, specifically, those aged 50-69 years were regarded as the optimal age group for screening. Nine of 23 guidelines recommended against an upper age limit for breast cancer screening.
Background The variation in breast cancer incidence rates across different regions may reflect disparities in breast cancer screening (BCS) practices. Understanding the factors associated with these screening behaviors is crucial for identifying modifiable elements amenable to intervention. This systematic review aims to identify common factors influencing BCS behaviors among women globally ...
Importance Breast cancer is a leading cause of premature mortality among US women. Early detection has been shown to be associated with reduced breast cancer morbidity and mortality. Objective To update the American Cancer Society (ACS) 2003 breast cancer screening guideline for women at average risk for breast cancer.. Process The ACS commissioned a systematic evidence review of the breast ...
Among women in the United States, breast cancer is the most common cancer, the second most common cause of death from cancer, and a leading cause of premature mortality as measured by the average and total years of life lost. 23 In 2019, the ACS estimates that there will be 268,600 cases of invasive breast cancer diagnosed in US women, and ...
This article is looking at literature on breast cancer screening. Being the most common cancer worldwide and a. leading cause of death, screening asymptotic women leads to early detec tion hence ...
Background This scoping review aimed to identify and present the evidence describing key motivations for breast cancer screening among women aged ≥ 75 years. Few of the internationally available guidelines recommend continued biennial screening for this age group. Some suggest ongoing screening is unnecessary or should be determined on individual health status and life expectancy. Recent ...
The breast cancer paradox: a systematic review of the association between area-level deprivation and breast cancer screening uptake in Europe. Cancer Epidemiol. 2019;60:77-85.
This systematic review reports that among US women at average risk for breast cancer, mammographic screening is associated with reduction in breast cancer ... Gierisch JM, et al. Benefits and Harms of Breast Cancer Screening: A Systematic Review. JAMA. 2015;314(15):1615-1634. doi:10.1001 ... AI in Clinical Practice Art and Images in ...
Breast cancer is the most common malignancy diagnosed in the female population worldwide and the leading cause of death among perimenopausal women. Screening is essential, since earlier detection in combination with improvements in breast cancer treatment can reduce the associated mortality. The aim of this study was to review and compare the recommendations from published guidelines on breast ...
The risk of breast cancer in women with initial exposure develops 8 years after radiation and increases over 25 years (Kumar V, Cotran RS, 2013). doing so (eg breast cancer screening) will lead to ...
Introduction. This systematic evidence review is an update of evidence for the U.S. Preventive Services Task Force (USPSTF) recommendation on breast cancer screening for average-risk women ().In 2002, based on results of a prior review (2, 3), the USPSTF recommended mammography screening, with or without clinical breast examination (CBE), every 1 to 2 years for women age 40 years and older.
e18761 Background: Among all types of cancer, breast cancer (BC) is the most diagnosed cancer in women and is the leading cause of cancer-related deaths in females globally. Developing countries possess approximately 60% to 70% of all worldwide breast cancer deaths. Despite the increasing efforts to expand awareness and encourage screening, breast cancer is still the most frequently diagnosed ...
Uncertain evidence. Globally, the US may be an outlier in making a strong recommendation to start population mammography screening at age 40 rather than age 50.10 11 12 The task force's evidence report found uncertain evidence of a potential mortality benefit in women aged 40-49: the 95% confidence interval spanned from six more deaths to 89 fewer deaths per 100 000 screened.3 None of the ...
The benefits and harms of breast cancer screening: an independent review. Brit J Cancer. 2013;108(11):2205-40. Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the US Preventive Services Task Force.
Screening is a key component of breast cancer early detection programs that can considerably reduce relevant mortality rates. The purpose of this study was to determine the breast cancer screening ...
Breast cancer incidence and mortality rates are increasing in the Arab world and the involved women are often diagnosed at advanced stages of breast cancer. This literature review explores factors influencing Arab women's breast cancer screening behavior. Searched databases were: Medline, PubMed, Cochrane Database of Systematic Reviews, CINAHL ...
Background Breast cancer clinical practice guidelines (CPGs) offer evidence-based recommendations to improve quality of healthcare for patients. Suboptimal compliance with breast cancer guideline recommendations remains frequent, and has been associated with a decreased survival. The aim of this systematic review was to characterize and determine the impact of available interventions to ...
With breast cancer being one of the most widespread causes of death for women, there is an unmet need for its early detection. For this purpose, we propose a non-invasive approach based on X-ray scattering. We measured samples from 107 unique patients provided by the Breast Cancer Now Tissue Biobank, with the total dataset containing 2958 entries. Two different sample-to-detector distances, 2 ...
Mataoui F. Correlates of breast cancer screening in Arab Muslim Women living in the United States. ... et al. Evaluating knowledge, attitudes, and practices toward breast cancer and breast cancer screening among females in Arab countries of the Middle East: A systematic literature review. JCO 2023;41(16_suppl):e18761-e18761;
The variation in breast cancer incidence rates across different regions may reflect disparities in breast cancer screening (BCS) practices. Understanding the factors associated with these screening behaviors is crucial for identifying modifiable elements amenable to intervention. ... Ahmadian M, Samah AA. A literature review of factors ...
Social needs inhibit receipt of timely medical care. Social needs screening is a vital part of comprehensive cancer care, and patient navigators are well-positioned to screen for and address social needs. This mixed methods project describes social needs screening implementation in a prospective pragmatic patient navigation intervention trial for minoritized women newly diagnosed with breast ...
Breast cancer care is a costly global health issue where effective management depends on early detection and treatment. A breast cancer diagnosis can result in financial catastrophe especially in low- and middle-income countries (LMIC). Large inequities in breast cancer care are observed and represent a global challenge to caregivers and patients.